The Correlation of Vegetative and Generative Characters of Duku (Lansium Domesticum Corr.) Accession in Banyuasin Regency, South Sumatra
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download Article (PDF)
Advances in Social Science, Education and Humanities Research, volume 565 Proceedings of the International Conference on Education Universitas PGRI Palembang (INCoEPP 2021) Free School Leadership Meilia Rosani1, Misdalina1*), Tri Widayatsih1 1Universitas PGRI Palembang, Indonesia *Corresponding author. Email: [email protected] ABSTRACT The introduction of free schools needs to be dealt with seriously by the leader. Seriousness is shown by his ability to lead. Leadership is required so that the introduction of free schools can be guided and targets can be accomplished quickly and efficiently. The purpose of this study was to decide how free school leadership is in the Musi Banyuasin Regency (MUBA). This research uses a qualitative approach to the case study process. The data collection technique was conducted by interviewing, analyzing and recording the validity of the data used in the triangulation process. The results show that free school leadership in the MUBA district is focused on coordination, collaboration, productivity in the division of roles, the arrangement of the activities of the management team members in an integrated and sustainable manner between the district management team and the school. Keywords: Leadership, Cohesion, Performance, Sustainability 1. INTRODUCTION Regional Governments, that education and the enhancement of the quality of human resources in the The Free School that is introduced in MUBA regions are also the responsibility of the Regional Regency is a program of the MUBA Regency Government. In addition to this clause, the leaders of the Government that has been implemented since 2003. MUBA District Government, along with stakeholders Until now, the software has been running well. -
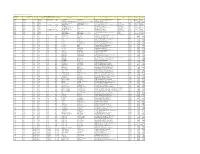
Colgate Palmolive List of Mills As of June 2018 (H1 2018) Direct
Colgate Palmolive List of Mills as of June 2018 (H1 2018) Direct Supplier Second Refiner First Refinery/Aggregator Information Load Port/ Refinery/Aggregator Address Province/ Direct Supplier Supplier Parent Company Refinery/Aggregator Name Mill Company Name Mill Name Country Latitude Longitude Location Location State AgroAmerica Agrocaribe Guatemala Agrocaribe S.A Extractora La Francia Guatemala Extractora Agroaceite Extractora Agroaceite Finca Pensilvania Aldea Los Encuentros, Coatepeque Quetzaltenango. Coatepeque Guatemala 14°33'19.1"N 92°00'20.3"W AgroAmerica Agrocaribe Guatemala Agrocaribe S.A Extractora del Atlantico Guatemala Extractora del Atlantico Extractora del Atlantico km276.5, carretera al Atlantico,Aldea Champona, Morales, izabal Izabal Guatemala 15°35'29.70"N 88°32'40.70"O AgroAmerica Agrocaribe Guatemala Agrocaribe S.A Extractora La Francia Guatemala Extractora La Francia Extractora La Francia km. 243, carretera al Atlantico,Aldea Buena Vista, Morales, izabal Izabal Guatemala 15°28'48.42"N 88°48'6.45" O Oleofinos Oleofinos Mexico Pasternak - - ASOCIACION AGROINDUSTRIAL DE PALMICULTORES DE SABA C.V.Asociacion (ASAPALSA) Agroindustrial de Palmicutores de Saba (ASAPALSA) ALDEA DE ORICA, SABA, COLON Colon HONDURAS 15.54505 -86.180154 Oleofinos Oleofinos Mexico Pasternak - - Cooperativa Agroindustrial de Productores de Palma AceiteraCoopeagropal R.L. (Coopeagropal El Robel R.L.) EL ROBLE, LAUREL, CORREDORES, PUNTARENAS, COSTA RICA Puntarenas Costa Rica 8.4358333 -82.94469444 Oleofinos Oleofinos Mexico Pasternak - - CORPORACIÓN -

Food Security and Environmental Sustainability on the South Sumatra Wetlands, Indonesia Syuhada, A.*1, M
Sys Rev Pharm 2020; 11(3): 457 464 A multifaceted review journal in the field of pharmacy E-ISSN 0976-2779 P-ISSN 0975-8453 Food Security and Environmental Sustainability on the South Sumatra Wetlands, Indonesia Syuhada, A.*1, M. Edi Armanto2, A. Siswanto3, M. Yazid4, Elisa Wildayana5 1* Environmental Study Program, Postgraduate Program, Sriwijaya University, Jln Raya Palembang-Prabumulih KM 32, Indralaya Campus, South Sumatra, Indonesia 2.4.5 Faculty of Agriculture, Sriwijaya University, Jln Raya Palembang-Prabumulih KM 32, Indralaya Campus, South Sumatra, Indonesia 3 Faculty of Engineering, Sriwijaya University, Jln Raya Palembang-Prabumulih KM 32, Indralaya Campus, South Sumatra, Indonesia. Article History: Submitted: 25.12.2019 Revised: 22.02.2020 Accepted: 10.03.2020 ABSTRACT The research aimed to analyze food security and environmental continuous rice-based; extensive perennial-based crops; and vegetable- sustainability in the South Sumatra wetlands. This research conducted on based crops. The main typology was dominated by continuous rice-based the wetlands of Banyuasin Regency, By Using the Survey method system taking around 197.961 ha or 58.50% and the lowest level was interview with respondents, guided by a questionnaire through the Focus shown by vegetable-based crops around 20.998 ha or 6.20%. Group Discussion (FGD). All data collected, by analyzed with SPSS Keywords: Food security, environmental sustainability, wetlands program. The results showed that plants planted in wetlands vary widely, Correspondance: such as rice, corn, soy, sweet potatoes, nuts, and cassava. The diversity of Syuhada, A commodities is cultivated on high enough wetlands; they seek food Environmental Study Program, Postgraduate Program, farming in order to meet their own subsistence with little agricultural input, Sriwijaya University, making it relatively difficult for crops to be able to produce optimally. -

The Effectiveness of Free Education Policy in Indonesia (A Case Study on the District of Musi Banyuasin South Sumatera)
The Effectiveness of Free Education Policy in Indonesia (A Case Study on the district of Musi Banyuasin South Sumatera) Dr. Muhammad Iqbal Department of Psychology, Mercu Buana University Jakarta Consultant of Bappenas Jakarta, 2011 Email : [email protected] Abstract This study aims to determine the effectiveness of the free educational program that has been conducted in Indonesian local government. This study investigates a case study on the implementation of free education in the district of Musi Banyuasin South Sumatera. The research was conducted qualitatively by analyzing secondary data and conduct interviews with stake holders whom are the executive of education from the Department of Education, the Principal and parents. From the research, it was found that the implementation of free education in Indonesia is still a political commodity, free education policy in practice still have problems and weaknesses. The constraints faced by schools is the budget provided by the local government is the cost of education and minimum service standards budget given are not payed monthly, but given every 6months which causes problems and obstacles in school operations, which then affects the school’s ability to thrive and compete with other schools. In the implementation of free education, the school is still collects school tuition fee from the parents through the school committee, this is because it takes a very large budget. Free schools policy also has psychological implications, where students and parents tend to be less concerned about the needs of the school, because they think everything is free, so the students have low interest and motivation, causing them to change schools freely because of the free education policy. -

Biodiversity Assessment Study: Avifauna
BIODIVERSITY ASSESSMENT STUDY: AVIFAUNA AT BUNGIN FIELD DEVELOPMENT PROJECT AREA SOUTH JAMBI B BLOCK CONOCOPHILLIPS (SOUTH JAMBI) LTD. BY JARWADI B HERNOWO DERA SYAFRUDIN December 2011 ConocoPhillips (South Jambi) Ltd. Ratu Prabu 2 Building Jl. TB. Simatupang Kav. 1-B Cilandak Timur, Jakarta 12560 Telp. (021) 78541000, Fax. (021) 7819910 Summary SUMMARY Biodiversity Assessment Survey for avifauna in Bungin Field Development project area of ConocoPhillips (South Jambi) Ltd. is conducted to collect and analyze data on species diversity, abundance and community structure as well as conservation status category. Based on the survey result, the total number of bird species identified in the study area are 72 species. Since, the diversity of birds has correlation with habitat types, the avifauna survey is designed in different types of habitat found in the study area . There are eight of habitat types determined for the avifauna survey at Bungin area, which include (i) oil palm plantation, (ii) mixed rubber plantation, (iii) shrubs, (iv) secondary growth, (v) pocket of degraded remnant forest, (vi) riparian forest, (vii) timber estate plantation & pocket remnant forest and (viii) logged over forest. The highest number of bird species are found at timber estate plantation and pocket remnant forest. Meanwhile the lowest bird diversity is observed in shrubs area. With regards to conservation status, from the total bird species identified, there are 14 species classified as protected by Government of Indonesia (GOI - Government Regulation 7/1999); -

Poverty Reduction in Regencies/Municipalities in South Sumatra Province
Society, 8 (2), 581-595, 2020 P-ISSN: 2338-6932 | E-ISSN: 2597-4874 https://society.fisip.ubb.ac.id Poverty Reduction in Regencies/Municipalities in South Sumatra Province Siti Rohima 1, Liliana 1, and Aning Kesuma Putri 2,* 1 Department of Economics, Faculty of Economics, Universitas Sriwijaya, 30662, South Sumatra Province, Indonesia 2 Department of Economics, Faculty of Economics, Universitas Bangka Belitung, 33172, Bangka Belitung Islands Province, Indonesia * Corresponding Author: [email protected] ARTICLE INFO ABSTRACT Publication Info: Local Government expenditure is budgeting for all government Research Article needs and activities and managed under the authority of provinces, regencies, and municipalities through their respective regional heads. Well-targeted Local Government How to cite: expenditure optimization has a significant impact on the Rohima, S., Liliana, L., & Putri, regional economy. This research aims to determine poverty A. K. (2020). Poverty Reduction reduction in regencies/municipalities in South Sumatra in Regencies/Municipalities in Province, Indonesia, by examining the variable’s impact of South Sumatra Province. Society, social assistance expenditure, capital expenditure, and local 8(2), 581-595. revenue on poverty. The data used are primary and secondary data obtained from 15 regencies/municipalities in South DOI: 10.33019/society.v8i2.215 Sumatra Province during the 2010-2018 periods. The analysis technique uses in this research were Poverty Mapping with Klassen Typology and Multiple Linear Regression (MLR). Copyright © 2020. Owned by Using the Klassen typology for poverty mapping in South Author(s), published by Society Sumatra Province obtained four regional classifications (quadrant) based on poverty and economic growth: quadrant I (developed and fast-growing region), quadrant II (developed but depressed region), quadrant III (developing region), and This is an open-access article. -

South Sumatra - West Java Phase II Gas Pipeline Project
Land Acquisition and Resettlement Plan Resettlement Plan Document Stage: Revised Project Number: 39928 July 2006 INO: South Sumatra - West Java Phase II Gas Pipeline Project Prepared by PT. Perusahaan Gas Negara Tbk (PGN) The resettlement plan is a document of the borrower. The views expressed herein do not necessarily represent those of ADB’s Board of Directors, Management, or staff, and may be preliminary in nature. ABBREVIATIONS ADB - Asian Development Bank AH - Affected Household AP - Affected Person BPN - Badan Pertanahan Nasional DKI - Daerah Khusus Ibukota CDP - Community Development Program EIA - Environmental Impact Assessment GOI - Government of the Republic of Indonesia KEPPRES - Keputusan Presiden KUD - Koperasi Unit Desa LARP - Land Acquisition and Resettlement Plan LKMD - Lembaga Ketahanan Masyarakat Desa NGO - Non Government Organization NJOP - Nilai Jual Objek Pajak PERPRES - Peraturan Presiden PGN - PT Perusahaan Gas Negara (Persero) Tbk PU - Pekerjaan Umum PKBL - Program Kemitraan dan Bina Lingkungan RoW - Right of Way SEIA - Summary Environmental Impact Assessment SES - Socio Economic Survey SSWJ - South Sumatra West Java SSWJ phase II South Sumatra West Java Gas Transmission Project phase II WEIGHTS AND MEASURES mmscfd - million standard cubic feet per day ha - Hectare km - Kilometer m - Meter m2 - square meter -ii- GLOSSARY Adat land Land held by customary community Affected Persons,or People (households) who, on account of the project, stand to lose households all or part of their physical and non-physical assets, including homes, communities, productive lands, resources such as forests, range lands, fishing areas, or important cultural sites, commercial properties, tenancy, income-earning opportunities, social and cultural networks and activities. Walikota/Regent Head of Kabupaten / District / Regency. -

Effect of Farmers' Characteristics, Information Sources, and Information Quality on Agriculture Risk Communication
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by GSSRR.ORG: International Journals: Publishing Research Papers in all Fields International Journal of Sciences: Basic and Applied Research (IJSBAR) ISSN 2307-4531 (Print & Online) http://gssrr.org/index.php?journal=JournalOfBasicAndApplied --------------------------------------------------------------------------------------------------------------------------- Effect of Farmers' Characteristics, Information Sources, and Information Quality on Agriculture Risk Communication Ali Alamsyah Kusumadinataa*, Sumardjob , Dwi Sadonoc , Burhanuddind aDepartment of Communication, Faculty of Social and Political Sciences, UNIDA, Bogor b,cDepartment of Communication Science and Community Development, Faculty of Human Ecology, IPB, Bogor dDepartment of Agribusiness, Faculty of Economics and Management, IPB, Bogor aEmail:[email protected] bEmail: [email protected] cEmail: [email protected] dEmail: [email protected] Abstract Risk communication is the exchange of information to increase the weight of managed farms. The weak ability of risk communication is influenced by the weak information received by farmers. This research was conducted in two districts of Banyuasin and Ogan Ilir in South Sumatra Province with a gradual sampling technique. The data in this sample amounted to 294 respondents. Respondents taken were respondents who were members of farmer groups. Data analysis was performed by frequency distribution and using SmartPLS software version 3.2.9. The results showed that farmers are categorized as 47th years old young adult farmers with 12 years of compulsory education level, ownership area of less than 2 ha, and employment status are farmers. As for those who influence the ability of farmers to communicate risk is the characteristics of farmers, information sources, and information quality. -

Revisiting the REDD+ Experience in Indonesia Lessons from National, Subnational and Local Implementation
CIFOR infobriefs provide concise, accurate, peer-reviewed information on current topics in forest research No. 314, December 2020 DOI: 10.17528/cifor/007880 | cifor.org Revisiting the REDD+ experience in Indonesia Lessons from national, subnational and local implementation Sandy Nofyanza, Moira Moeliono, Vivi Selviana, Bimo Dwisatrio, Nining Liswanti, Ade Ryane Tamara and Mella Komalasari Key messages • In Indonesia, early involvement and support for Reducing Emissions from Deforestation and Forest Degradation (REDD+) has led to numerous achievements, but progress has been slower than anticipated. • National and subnational REDD+ initiatives are susceptible to political turnover at each election cycle. To ensure its longevity, REDD+ needs to be embedded in national and regional laws, regulations, institutions and other state devices. • REDD+ institutionalization in Indonesia has focused on technicalities rather than on directly addressing socioeconomic and political drivers of deforestation and forest degradation. The rate of deforestation has decelerated enough to result in two REDD+ payments. However, transformational change in the forestry and broader land-use sector has not progressed far enough. • REDD+ is inherently multilevel and multisectoral. However, much information, action, knowledge exchange and decision making on REDD+ is concentrated within relatively few organizations. Transformational change requires that other stakeholders and sectors that impact forests get involved. Introduction announced that Indonesia would receive, as its first results-based REDD+ payment, USD 56 million for In this brief we aim to highlight several lessons from the reducing 4.8 MtCO2 emissions in 2017 against the experience of Reducing Emissions from Deforestation 2006–2016 historical baseline (Seymour, 2019). More and forest Degradation (REDD+) in Indonesia and recently, Indonesia received another performance- explore relevant opportunities for future forest-based based payment – USD 103 million from the Green climate mitigation and adaptation initiatives. -

An Updated Checklist of the Mosquitoes from South Sumatra Province with a New Record of Aedes (Downsiomyia) Pexus Colless, 1958 (Diptera: Culicidae) in Indonesia
Treubia 44: 29–46, December 2017 AN UPDATED CHECKLIST OF THE MOSQUITOES FROM SOUTH SUMATRA PROVINCE WITH A NEW RECORD OF AEDES (DOWNSIOMYIA) PEXUS COLLESS, 1958 (DIPTERA: CULICIDAE) IN INDONESIA Sidiq Setyo Nugroho*1, Mujiyono1, Triwibowo Ambar Garjito1, Riyani Setiyaningsih1, Siti Alfiah1, Yahya2, Anif Budiyanto2 and Lasbudi Pertama Ambarita2 1Institute of Vector and Reservoir Control Research and Development, National Institute of Health Research and Development, Ministry of Health Indonesia 2Vector-borne Disease Research Unit of Baturaja, National Institute of Health Research and Development, Ministry of Health Indonesia *Corresponding author: [email protected] Received: 29 May 2017; Accepted: 23 November 2017 ABSTRACT Data of mosquito fauna is important to be known as basic effort in vector mosquito control. It is necessary to update the data from time to time. The effort of updating the mosquito fauna was started from South Sumatra Province. Amount of 2,784 mosquito specimens were examined. The result showed there are 62 species of mosquitoes from South Sumatra Province and they belong to 10 genera. One species of culicid mosquito were recorded for the first time from Indonesia, namely Aedes (Downsiomyia) pexus and six other species were first recorded on Sumatra Island. These species are now included in the Sumatran Culicidae checklist. Key words: Indonesia, mosquito fauna, new species record, South Sumatra INTRODUCTION Many species of Culicidae family, or known as mosquitoes, have been recognised as vectors of medically important pathogens and parasites such as viruses, protozoans, and nematodes that are causing diseases in human. Culicidae family includes two sub-families, 44 genera, 145 subgenera and 3,490 species worldwide (Harbach & Howard 2007). -
Peran Kader Sebagai Tenaga Pelaksana Eliminasi Program Pemberian Obat Pencegahan Massal Limfatik Filariasis Tahap Iii Di Kabupaten Banyuasin
SPIRAKEL, Vol. 12 No.1, Juni 2020: 15-26 Peran Kader sebagai … (Indah, Reni) DOI:https://doi.org/10.22435/spirakel.v12i1.3313 PERAN KADER SEBAGAI TENAGA PELAKSANA ELIMINASI PROGRAM PEMBERIAN OBAT PENCEGAHAN MASSAL LIMFATIK FILARIASIS TAHAP III DI KABUPATEN BANYUASIN Indah Margarethy1*, Reni Oktarina2 1Balai Penelitian dan Pengembangan Kesehatan Baturaja Jl. A.Yani KM.7 Kemelak Baturaja, 32111, Kabupaten Ogan Komering Ulu, Sumatera Selatan, Indonesia 2 Badan Penelitian dan Pengembangan Daerah Provinsi Sumatera Selatan, Jl. Demang Lebar Daun No. 4864, Palembang, 30137, Kota Palembang Sumatera Selatan, Indonesia Abstract Banyuasin Regency is a filariasis endemic area in South Sumatra Province with total 90 chronic cases reported in 2014 while in 2018 decreased to 46 cases. Strategic coordination and good cooperation from various parties are needed to achieve the goals of elimination of lymphatic filariasis, one of them is by involving community as cadres of community drug distributors (CDDs) in mass drug administration (MDA). This study aimed to determine the role of CDDs in (MDA) filariasis phase III in Banyuasin Regency. Data collection was carried out through in- depth interviews with selected informants, namely: Filariasis Program director of the Banyuasin District Health Office, Filariasis Program director at puskesmas in Banyuasin Regency, village midwives, and cadres. This type of research is qualitative so informants are adjusted to the data requirements. The data obtained were analyzed using content analysis. The results showed the implementation of phase III MDA filariasis in Banyuasin Regency hadn’t proceeded according to the procedure. In the implementation, the CDDs has a role in helping health workers to collect data on target populations and people who get drugs, socialize MDA activities to the people in their areas and distribute medicine at treatment posts and house to house. -
Part 3 STUDY for MUSI RIVER BASIN
Part 3 STUDY FOR MUSI RIVER BASIN The Project for Assessing and Integrating Climate Change Impacts into the Water Resources Management Plans for Brantas and Musi River Basins Final Report (Water Resources Management Plan) Main Report CHAPTER 7 COLLECTION AND COMPILATION OF INFORMATION AND DATA 7.1 Natural Condition of Project Area 7.1.1 Topography and Geology (1) Topography The Musi River is the largest river in Sumatra flowing down from west to east in South Sumatra Province which has the fourth largest catchment area of 59,942 km2 in Indonesia, and it is approximately 640km long. The average bed slope widely varies from upstream (1/100 - 1/200) to downstream (1/10,000) around Palembang, and becomes gentler (1/20,000) toward the coastal areas. The topography of the Musi River basin can be mainly classified into five zones as shown in Figure 7.1.1. The mountainous zone is distributed only to the west-southwest-south region of the basin under the influence of the prominent geological structure that indicates the northwest-southeast strike. The remaining 60% of the basin excluding the mountainous zone and its adjacent piedmont zone, is occupied by central plains, inland wetlands, and coastal plains. Table 7.1.1 Topographic Zones of Musi River Basin Zone Distribution Area Topographical Feature South-southwest-west of Mountainous Zone Valley, highland, and volcano the basin Between mountainous Undulating hills (Distribution with a width of about 40km to Piedmont Zone zone and central plains the northwest-southeast direction) Between piedmont zone Can be classified into three: plateau, floodplain, and river Central Plains and coastal plains levees Mainly along the rivers of Inland Wetlands Natural levee and marsh the downstream Coastal lowlands and delta lowlands covered with peat Coastal Plains Coastal and around delta swamp forest Source: JICA Project Team 2 (2) Geology A geological map of the Musi River basin is shown in Figure 7.1.1.