Effect of Addition of Nacl on the Functional Properties Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Effect of Irradiation of the PEM of 1.531211SMJ29 Jeewanu with Clinical Mercury Lamp and Sunlight on the Morphological Features of the Silicon Molybdenum Jeewanu
International Journal of Engineering Research and General Science Volume 4, Issue 4, July-August, 2016 ISSN 2091-2730 Effect of Irradiation of the PEM of 1.531211SMJ29 Jeewanu with Clinical Mercury Lamp and Sunlight on the Morphological Features of the Silicon Molybdenum Jeewanu Deepa Srivastava Department of Chemistry, S.S.Khanna Girls‘ Degree College, Constituent College of Allahabad University, Allahabad, Uttar Pradesh, India E- Mail – [email protected] Abstract— Sterilized aqueous mixture of ammonium molybdate, diammonium hydrogen phosphate, mineral solution and formaldehyde on exposure to sunlight results in the formation of self-sustaining coacervates which were coined as Jeewanu, the autopoetic eukaryote by Bahadur and Ranganayaki. Jeewanu have been analyzed to contain a number of compounds of biological interest. The presence of various enzyme like activities viz., phosphatase, ATP-ase, esterase, nitrogenase have been also been detected in Jeewanu mixture. Gáinti (2003) discussed that Jeewanu possesses a promising configuration similar to protocell-like model. Keywords— Autopoetic, eukaryote, Jeewanu, PEM, sunlight, mercury lamp, morphology, 1.531211SMJ29 INTRODUCTION Sterilized aqueous mixture of ammonium molybdate, diammonium hydrogen phosphate, mineral solution and formaldehyde on exposure to sunlight results in the formation of self-sustaining coacervates which were coined as Jeewanu, the autopoetic eukaryote by Bahadur, K and Ranganayaki, S. in 1970. [1] The photochemical, formation of protocell-like microstructures ―Jeewanu‖ in a laboratory simulated prebiotic atmosphere capable of showing multiplication by budding, growth from within by actual synthesis of material and various metabolic activities has been reported by Bahadur et al. [1, 2, 3, 4, 5, 7, 8] Jeewanu have been analyzed to contain a number of compounds of biological interest viz. -

Download Report 2010-12
RESEARCH REPORt 2010—2012 MAX-PLANCK-INSTITUT FÜR WISSENSCHAFTSGESCHICHTE Max Planck Institute for the History of Science Cover: Aurora borealis paintings by William Crowder, National Geographic (1947). The International Geophysical Year (1957–8) transformed research on the aurora, one of nature’s most elusive and intensely beautiful phenomena. Aurorae became the center of interest for the big science of powerful rockets, complex satellites and large group efforts to understand the magnetic and charged particle environment of the earth. The auroral visoplot displayed here provided guidance for recording observations in a standardized form, translating the sublime aesthetics of pictorial depictions of aurorae into the mechanical aesthetics of numbers and symbols. Most of the portait photographs were taken by Skúli Sigurdsson RESEARCH REPORT 2010—2012 MAX-PLANCK-INSTITUT FÜR WISSENSCHAFTSGESCHICHTE Max Planck Institute for the History of Science Introduction The Max Planck Institute for the History of Science (MPIWG) is made up of three Departments, each administered by a Director, and several Independent Research Groups, each led for five years by an outstanding junior scholar. Since its foundation in 1994 the MPIWG has investigated fundamental questions of the history of knowl- edge from the Neolithic to the present. The focus has been on the history of the natu- ral sciences, but recent projects have also integrated the history of technology and the history of the human sciences into a more panoramic view of the history of knowl- edge. Of central interest is the emergence of basic categories of scientific thinking and practice as well as their transformation over time: examples include experiment, ob- servation, normalcy, space, evidence, biodiversity or force. -

Conference Report Highlights of the Meeting on “Horizontal Gene
ORIENTAL JOURNAL OF CHEMISTRY ISSN: 0970-020 X CODEN: OJCHEG An International Open Free Access, Peer Reviewed Research Journal 2013, Vol. 29, No. (4): Pg. 1687-1693 www.orientjchem.org Conference Report Highlights of the Meeting on “Horizontal Gene Transfer and the Last Universal Common Ancestor” Held at the Open University, Milton Keynes, UK SOHAN Jheeta Network of Researchers on Horizontal Gene Transfer and the Last Universal Common Ancestor, 1 Scott Hall Crescent, Leeds, LS7 3RB, U.K. *Corresponding author E-mail: [email protected] http://dx.doi.org/10.13005/ojc/290459 (Received: July 12, 2013; Accepted: August 04, 2013) ABstract This paper summarises the oral presentations, their outcomes and conclusions drawn from the meeting entitled: ‘Horizontal Gene transfer and the Last Universal Common Ancestor’ which was held at the Open University, Milton Keynes, UK, on the 5th and the 6th September 2013. Key words: Horizontal gene transfer; HGT, Lateral gene transfer; LGT, Phylogenetic tree of life, Transformation; Gene transfer agent (GTA); Last universal common ancestor (LUCA) INTRODUCTION brought together an international gathering of scientists from Japan, India, Israel, Russia, USA, One of the first of its kind, this meeting, Canada, Germany, Denmark, Italy, Spain as well held on the 5th and the 6th September, was designed as participants from the UK (Figure 3). With such an to investigate and evaluate the importance of assortment of eminent researchers under one roof, ‘horizontal gene transfer’ (HGT) during the period of the meeting was both energetic and engaging and the ‘last universal common ancestor’ (LUCA) prior generated a lively debate and exchange of ideas. -
Bani of Bhagats-Part II.Pmd
BANI OF BHAGATS Complete Bani of Bhagats as enshrined in Shri Guru Granth Sahib Part II All Saints Except Swami Rama Nand And Saint Kabir Ji Dr. G.S. Chauhan Publisher : Dr. Inderjit Kaur President All India Pingalwara Charitable Society (Regd.) Amritsar-143001 Website:www.pingalwara.co; E-mail:[email protected] BANI OF BHAGATS PART : II Author : G.S. Chauhan B-202, Shri Ganesh Apptts., Plot No. 12-B, Sector : 7, Dwarka, New Delhi - 110075 First Edition : May 2014, 2000 Copies Publisher : Dr. Inderjit Kaur President All India Pingalwara Charitable Society (Regd.) Amritsar-143001 Ph : 0183-2584586, 2584713 Website:www.pingalwara.co E-mail:[email protected] (Link to download this book from internet is: pingalwara.co/awareness/publications-events/downloads/) (Free of Cost) Printer : Printwell 146, Industrial Focal Point, Amritsar Dedicated to the sacred memory of Sri Guru Arjan Dev Ji Who, while compiling bani of the Sikh Gurus, included bani of 15 saints also, belonging to different religions, castes, parts and regions of India. This has transformed Sri Guru Granth Sahib from being the holy scripture of the Sikhs only to A Unique Universal Teacher iii Contentsss • Ch. 1: Saint Ravidas Ji .......................................... 1 • Ch. 2: Sheikh Farid Ji .......................................... 63 • Ch. 3: Saint Namdev Ji ...................................... 113 • Ch. 4: Saint Jaidev Ji......................................... 208 • Ch. 5: Saint Trilochan Ji .................................... 215 • Ch. 6: Saint Sadhna Ji ....................................... 223 • Ch. 7: Saint Sain Ji ............................................ 227 • Ch. 8: Saint Peepa Ji.......................................... 230 • Ch. 9: Saint Dhanna Ji ...................................... 233 • Ch. 10: Saint Surdas Ji ...................................... 240 • Ch. 11: Saint Parmanand Ji .............................. 244 • Ch. 12: Saint Bheekhan Ji................................ -

Experimental Confirmation of the Reich Orgone Accumulator Thermal Anomaly
Research EXPERIMENTAL CONFIRMATION OF THE REICH ORGONE ACCUMULATOR THERMAL ANOMALY by James DeMeo, Ph.D. ABSTRACT EXperimental inVestigations Were undertaken bY the author, of the thermal anomalY (To-T) inside the orgone energy accumulator (ORAC), a phenomenon firstlY obserVed bY the late Dr. Wilhelm Reich, Who inVented the ORAC deVice. This thermal anomalY, bY the theorY of Reich, is produced from the rarified motional-pulsating orgone energy continuum Which is concentrated inside the ORAC, producing a frictional thermal heating of the air. Discussion is giVen on the eXperimental proofs standing behind Reich’s theorY and claims, draWing attention to similar concepts in the modern sciences. The orgone energY is similar in manY respects to the older luminiferous cosmic ether in that it fills all space, but also fulfills the role of an atmospheric-biological life-energy, in that it is pulsatorY and eXcitable, and charges liVing tissues. In the To-T eXperiment, air temperature Was measured inside the upper part of a 10 cm cubical ORAC, and contrasted to the temperature Within a thermallY-balanced but non-orgone-accumulating Control enclosure, folloWing the protocols of Reich. EXceptional care Was taken in the construction of the apparatus and in control procedures, With instrumentation calibrated doWn to ~0.002˚C. The eXperiment Was undertaken in a Well-Ventilated but fullY dark-shaded outdoor thermal shelter speciallY constructed for the eValuations. Under the optimal conditions for ORAC functioning (i.e., loW humiditY, light or no Winds, clear skies), a cYclical positiVe thermal anomalY Was sYstematicallY detected, With an aVerage of +0.13˚C differential in tWo 10-daY eXperimental runs presented here, With maXima peaking dailY around +0.5˚C Warmer than the Control, and minima at around -0.1˚C. -

Interstellar Index: Papersinterstellarindex
Interstellar Index: papersInterstellarindex http://www.interstellarindex.com/technical-papers/ Interstellarindex The reliable web site for interstellar information designed and managed by www.I4IS.org Papers On this page we list every technical paper known to us that has been published on interstellar studies or related themes. This includes papers on: Spacecraft concepts, designs and mission architectures, robotic and manned, which may be relevant to interstellar flight; Space technologies at all readiness levels (TRL1-9), especially in propulsion; General relativity and quantum field theory as applied to proposed warp drives and wormholes in spacetime; Any other breakthrough physics concepts applicable to propulsion; Nearby mission targets, particularly exoplanets within 20 light-years; Sociology, politics, economics, philosophy and other interstellar issues in the humanities; The search for extraterrestrial intelligence (SETI) and related search and messaging issues. A few pieces of information are missing, as shown by queries {in curly brackets}. Please be patient while we fill in these gaps. * * * 2013 Baxter, S., “Project Icarus: Interstellar Spaceprobes and Encounters with Extraterrestial Intelligence”, JBIS, 66, no.1/2, pp.51-60, Jan./Feb. 2013. Benford, J., “Starship Sails Propelled by Cost-Optimized Directed Energy”, JBIS, 66, no.3/4, pp.85-95, March/April 2013. Beyster, M.A., J. Blasi, J. Sibilia, T. Zurbuchen and A. Bowman, “Sustained Innovation through Shared Capitalism and Democratic Governance”, JBIS, 66, no.3/4, pp.133-137, March/April 2013. Breeden, J.L., “Gravitational Assist via Near-Sun Chaotic Trajectories of Binary Objects”, JBIS, 66, no.5/6, pp.190-194, May/June 2013. Cohen, M.M., R.E. -

Histochemical Localisation of RNA-Like Material in Photochemically
OPEN ACCESS Int. Res. J. of Science & Engineering, 2013, Vol.1 (1): 1-4 ISSN: 2322-0015 ORIGINAL ARTICLE . Histochemical localisation of RNA-like material in photochemically formed self-sustaining, abiogenic supramolecular assemblies ‘Jeewanu’ Gupta VK1* and Rai RK2 1Department of Zoology, C.M.D. Post Graduate College, Bilaspur (Chhattisgarh) India 2Department of Zoology, Govt. Degree College, Dabhara, Janjgir-Champa (Chhattisgarh) India. *Corresponding author Email – [email protected] ABSTRACT KEYWORDS Sunlight exposed sterilised aqueous mixture of some inorganic and organic Jeewanu, substances showed the photochemical formation of self-sustaining, supra- Self-sustaining, molecular assemblies ‘Jeewanu’. The microscopic observation (Optical, SCM and Supramolecular, TEM) showed that they possess an ordered structural configuration. They are Abiogenesis, INT. RES. J. OF SCIENCE J. & ENGINEERINGINT. RES. capable of showing various properties of biological order viz. multiplication by Protocells budding, growth from within and metabolic activities in them. The histochemical investigation of Jeewanu showed the presence of RNA-like material. The Abiogenesis of RNA-like substances in Jeewanu mixture supports RNA world theory suggesting its primitively. © 2013| Published by IRJSE INTRODUCTION inheritance and catalysis. RNA might have come first and catalyzed all necessary reactions necessary The emergence of the first cell on the Earth was for survival and replication (Gilbert, 1986; Joyce and the culmination of long history of prior chemical Orgel 1993; Orgel, 1994b). It was suggested that and physical process (Schrum et al., 2010). The RNA being more potent then DNA (Joyce, 1989; construction of protocell-like model with properties Smith and Szathmary, 1995). of biological order is significant in the study of origin of life. -

Wilhelm Reich, Biologist—A Selected Bibliography Abir-Am, Pnina. “The
Wilhelm Reich, Biologist—a selected bibliography Abir-am, Pnina. “The Discourse of Physical Power and Biological Knowledge in the 1930s: A Reappraisal of the Rockefeller Foundation’s ‘Policy in Molecular Biology’,” Social Studies of Science 12: 341-382 (1982). Acocella, Joan. “The Empty Couch,” New Yorker 8 May 2000, pp. 112-118. Ackert, Lloyd. Sergei Vinogradskii and the Cycle of Life: from the Thermodynamics of Life to Ecological Microbiology, 1850-1950 (New York: Springer, 2013). Adler, L.K. and T.G. Paterson, “Red Fascism: the Merger of Nazi Germany and Soviet Russia in the American Image of Totalitarianism, 1930s-1950s,” Amer. Histor. Rev. 75: 1046-1064 (1970). Alberts, Bruce. “The Cell as a Collection of Protein Machines: Preparing the Next Generation of Molecular Biologists,” Cell 92: 291–294 (6 February 1998). Allen, F. J. “What Is Life?” Proc. Birmingham Nat. Hist. Phil. Soc. 11: 44-67 (1899). Allen, Garland. Life Science in the Twentieth Century (Cambridge: Cambridge U. Press, 1975). Allen, Garland. Thomas Hunt Morgan: The Man and his Science (Princeton, NJ: Princeton U. Press, 1978). Allen, Garland. “Mechanism, Vitalism and Organicism in Late Nineteenth and Twentieth Century Biology: the Importance of Historical Context.” Stud. Hist. Phil. Biol. Biomed. Sci. 36: 261-283 (2005). Allen, Garland. “Rebel With Two Causes: Hans Driesch,” pp. 37-64 in Oren Harman and Michael Dietrich, eds., Rebels, Mavericks and Heretics in Biology (New Haven: Yale U. Press, 2008). Amsterdamska, Olga. “Stabilizing Instability: The Controversy over Cyclogenic Theories of Bacterial Variation during the Interwar Period,” J. Hist. Biol. 24: 191–222 (1991). Amsterdamska, Olga. “Medical and Biological Constraints: Early Research on Variation in Bacteriology,” Social Studies of Science, 17: 657-687 (1987). -

From Biophysics to Biology
Springer Series in Biophysics 19 Richard M. Epand Jean-Marie Ruysschaert Editors The Biophysics of Cell Membranes Biological Consequences Springer Series in Biophysics Volume 19 Series editor Boris Martinac [email protected] More information about this series at http://www.springer.com/series/835 [email protected] Richard M. Epand • Jean-Marie Ruysschaert Editors The Biophysics of Cell Membranes Biological Consequences 123 [email protected] Editors Richard M. Epand Jean-Marie Ruysschaert Biochemistry and Biomedical Sciences Sciences Faculty McMaster University Université Libre de Bruxelles Hamilton, ON, Canada Bruxelles, Belgium ISSN 0932-2353 ISSN 1868-2561 (electronic) Springer Series in Biophysics ISBN 978-981-10-6243-8 ISBN 978-981-10-6244-5 (eBook) DOI 10.1007/978-981-10-6244-5 Library of Congress Control Number: 2017952612 © Springer Nature Singapore Pte Ltd. 2017 This work is subject to copyright. All rights are reserved by the Publisher, whether the whole or part of the material is concerned, specifically the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfilms or in any other physical way, and transmission or information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed. The use of general descriptive names, registered names, trademarks, service marks, etc. in this publication does not imply, even in the absence of a specific statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use. The publisher, the authors and the editors are safe to assume that the advice and information in this book are believed to be true and accurate at the date of publication. -

Evaluation of Seeds of Celastrus Paniculatus Willd. – a Potential Medicinal Plant of Pharmacopoeia
Asian J. Exp. Sci., Vol. 27, No. 2, 2013; 1-2 Evaluation of seeds of Celastrus paniculatus Willd. – A potential medicinal plant of Pharmacopoeia Kaushal Kumar, S. G. Abbas and M.H. Siddiqui Faculty of Forestry, Birsa Agricultural University, Kanke, Ranchi-834006 Email: [email protected] Abstract : Celastrus paniculatus Willd. (Celastraceae) is a potential medicinal plant extensively used in ayurvedic medicinal systems. The seeds oil of these medicinal plants is used for the treatment of large numbers of diseases. The seeds of this plant were collected from four regions of Jharkhand state and the various constitution and oil were compared with the parameters described in the Ayurvedic pharmacopoeia of Govt. of India. The percentage of oil contents of Celastrus paniculatus Willd. ranges from 43.2 to 44.67 % while the contents of the oil mentioned in pharmacopeia is 45%. The similarities in other contents like foreign matter, total ash, acid insoluble ash, alcohol soluble extract and water soluble extract too is identical. It is, therefore, suggested that the Celastrus paniculatus Willd. should be protected and awareness should be generated amongst public private sector for the production of seed oil at cheap rate for medicinal use. Keywords: Celastrus paniculatus, Seed oil, Pharmacopoea, Quality evaluation Introduction Methodology of works Celastrus paniculatus Willd. is a large climbing The seeds of Celastrus paniculatus Willd were deciduous shrub, yellowish wood, with lenticelled collected from four different places from the district of branches. It belongs to family Celastraceae. The plant Ranchi (Jonha), Gumla (Bishunpur), Dumka (Baghraidih) mostly occurs in sub Himalayan tract up to 6000 ft. -
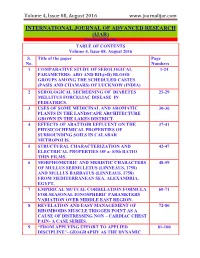
Table-Of-Contents4.Pdf
Volume 4, Issue 08, August 2016 www.journalijar.com INTERNATIONAL JOURNAL OF ADVANCED RESEARCH (IJAR) www.journalijar.com TABLE OF CONTENTS Volume 4, Issue 08, August 2016 S. Title of the paper Page No. Numbers 1 COMPARATIVE STUDY OF SEROLOGICAL 1-24 PARAMETERS: ABO AND RH((±D) BLOOD GROUPS AMONG THE SCHEDULED CASTES (PASIS AND CHAMARS) OF LUCKNOW (INDIA) 2 SEROLOGICAL SECREENING OF DIABETES 25-29 MELLITUS FORCELIAC DISEASE IN PEDIATRICS. 3 USES OF SOME MEDICINAL AND AROMATIC 30-36 PLANTS IN THE LANDSCAPE ARCHITECTURE GROWN IN THE LAKES DISTRICT. 4 EFFECTS OF ABATTOIR EFFLUENT ON THE 37-41 PHYSICOCHEMICAL PROPERTIES OF SURROUNDING SOILS IN CALABAR METROPOLIS. 5 STRUCTURAL CHARACTERIZATION AND 42-47 ELECTRICAL PROPERTIES OF a- S/Sb RATIO THIN FILMS. 6 MORPHOMETRIC AND MERISTIC CHARACTERS 48-59 OF MULLUS SERMULETUS (LINNEAUS, 1758) AND MULLUS BARBATUS (LINNEAUS, 1758) FROM MEDITERRANEAN SEA, ALEXANDRIA, EGYPT. 7 EMPIRICAL MUTUAL CORRELATION FORMULA 60-71 FOR SEASONAL IONOSPHERIC PARAMETERS VARIATION OVER MIDDLE EAST REGION. 8 REVELATION AND EASY MANAGEMENT OF 72-80 RHOMBOIDS MUSCLE TRIGGER POINT AS A CAUSE OF DISTRESSING NON – CARDIAC CHEST PAIN- A CASE SERIES. 9 ―FROM APPLYING EFFORT TO APPLIED 81-100 DISCIPLINE‖- GEOGRAPHY AS THE DYNAMIC Volume 4, Issue 08, August 2016 www.journalijar.com FIELD WITH TODAY‘S CONTEXTUALIZING ISSUES OF SOCIETY AND NATURE. 10 A CLOSER LOOK AT CATEGORIES OF 101-105 STEGANOGRAPHY TECHNIQUES. 11 PRIMARY NEROENDOCRINE SMALL CELL 106-108 CARCINOMA OF THE BREAST RARE PATHOLOGY- CASE REPORT. 12 THE LEGAL EFFECTS OF SUPERVISION OF THE 109-117 OFFICE OF FEDERAL FINANCIAL SUPERVISORY IN IRAQ -EGYPT, FRANCE. -

Life Sciences, 2018; 6 (4):877-884 Life Sciences ISSN:2320-7817(P) | 2320-964X(O)
International Journal of Int. J. of Life Sciences, 2018; 6 (4):877-884 Life Sciences ISSN:2320-7817(p) | 2320-964X(o) International Peer Reviewed Open Access Refereed Journal Original Article Open Access Cytochemical characterisation of photochemically formed, self- sustaining, abiogenic, protocell-like, supramolecular assemblies “Jeewanu” Gupta VK1* and Rai RK2 1Department of Zoology, C.M. Dubey Post Graduate College, Bilaspur (Chhattisgarh) India. 2Department of Zoology, Govt. Mahamaya College Ratanpur, Bilaspur (Chhattisgarh) India. *Corresponding author: Email - [email protected] & [email protected] | Mob. No. - 09424153429 Manuscript details: ABSTRACT Received: 19.10.2018 Sunlight exposed sterilised aqueous mixture of some inorganic and organic Accepted: 23.11.2018 substances reported by Bahadur and Ranganayaki provided an optimal Published: 22.12.2018 physico-chemical environment for the self-assembly of molecules and photochemical formation of self-sustaining, abiogenic, protocell-like model Editor: Dr. Arvind Chavhan ‘Jeewanu’. They possess an ordered structural configuration. They are capable of showing multiplication by budding, growth from within. The Cite this article as: histochemical findings showed that the acidic material-like substances Gupta VK and Rai RK (2018) were localised in the central region, basic material-like substances were Cytochemical characterisation of localised in extra central region while phospholipid-like substances were photochemically formed, self- present in outer limiting surface of Jeewanu. The presence of RNA-like sustaining, abiogenic, protocell-like, material is significant. In plausible prebiotic atmosphere the synthesized supramolecular assemblies protocell-like model similar to Jeewanu was photoautotrophic in nature. “Jeewanu”, Int. J. of. Life Sciences, They have an ability to convert solar energy into vital activities or useful Volume 6(4): 877-884.