The Distribution of T and B Lymphocyte Populations and MHC Class II Expression in Human Fetal and Postnatal Intestine
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Our Immune System (Children's Book)
OurOur ImmuneImmune SystemSystem A story for children with primary immunodeficiency diseases Written by IMMUNE DEFICIENCY Sara LeBien FOUNDATION A note from the author The purpose of this book is to help young children who are immune deficient to better understand their immune system. What is a “B-cell,” a “T-cell,” an “immunoglobulin” or “IgG”? They hear doctors use these words, but what do they mean? With cheerful illustrations, Our Immune System explains how a normal immune system works and what treatments may be necessary when the system is deficient. In this second edition, a description of a new treatment has been included. I hope this book will enable these children and their families to explore together the immune system, and that it will help alleviate any confusion or fears they may have. Sara LeBien This book contains general medical information which cannot be applied safely to any individual case. Medical knowledge and practice can change rapidly. Therefore, this book should not be used as a substitute for professional medical advice. SECOND EDITION COPYRIGHT 1990, 2007 IMMUNE DEFICIENCY FOUNDATION Copyright 2007 by Immune Deficiency Foundation, USA. Readers may redistribute this article to other individuals for non-commercial use, provided that the text, html codes, and this notice remain intact and unaltered in any way. Our Immune System may not be resold, reprinted or redistributed for compensation of any kind without prior written permission from Immune Deficiency Foundation. If you have any questions about permission, please contact: Immune Deficiency Foundation, 40 West Chesapeake Avenue, Suite 308, Towson, MD 21204, USA; or by telephone at 1-800-296-4433. -

Defining Natural Antibodies
PERSPECTIVE published: 26 July 2017 doi: 10.3389/fimmu.2017.00872 Defining Natural Antibodies Nichol E. Holodick1*, Nely Rodríguez-Zhurbenko2 and Ana María Hernández2* 1 Department of Biomedical Sciences, Center for Immunobiology, Western Michigan University Homer Stryker M.D. School of Medicine, Kalamazoo, MI, United States, 2 Natural Antibodies Group, Tumor Immunology Division, Center of Molecular Immunology, Havana, Cuba The traditional definition of natural antibodies (NAbs) states that these antibodies are present prior to the body encountering cognate antigen, providing a first line of defense against infection thereby, allowing time for a specific antibody response to be mounted. The literature has a seemingly common definition of NAbs; however, as our knowledge of antibodies and B cells is refined, re-evaluation of the common definition of NAbs may be required. Defining NAbs becomes important as the function of NAb production is used to define B cell subsets (1) and as these important molecules are shown to play numerous roles in the immune system (Figure 1). Herein, we aim to briefly summarize our current knowledge of NAbs in the context of initiating a discussion within the field of how such an important and multifaceted group of molecules should be defined. Edited by: Keywords: natural antibody, antibodies, natural antibody repertoire, B-1 cells, B cell subsets, B cells Harry W. Schroeder, University of Alabama at Birmingham, United States NATURAL ANTIBODY (NAb) PRODUCING CELLS Reviewed by: Andre M. Vale, Both murine and human NAbs have been discussed in detail since the late 1960s (2, 3); however, Federal University of Rio cells producing NAbs were not identified until 1983 in the murine system (4, 5). -
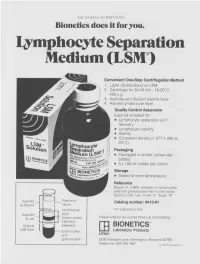
Lymphocyte Separation Medium (LSM
THE JOURNAL OF IMMUNOLOGY Bionetics does it for you. Lymphocyte Separation Medium (LSM wenient One-Step Centrifugation Method _ayer diluted blood on LSM. 2,entrifuge for 30-40 min., 18-20°C, ~.00 x g. ~,spirate and discard plasma layer -larvest lymphocyte layer. Quality Control Assurance Each lot is tested for: • Lymphocyte separation and recovery. • Lymphocyte viability. • Sterility. • Consistent density (1.077-1.080 at 20°C). Packaging • Packaged in amber, screw-cap bottles. • 5 x 100 ml bottles per carton. Storage. • Stored at room temperature. Reference Boyum, A. (1968): Isolation of mononuclear cells and granulocytes from human blood. Scand J. Clin. Lab. Invest. 21, Suppl. 97. Aspirate I IC[OI I IC~ LJI Catalog number: 8410-01 & discard serum Lymphocyte For Laboratory Use Aspirate layer & use (mononuclear Please write for our current Price List and Catalog. cells and Original platelets) ITi BIONETICS° LSM layer Erythrocytes Laboratory Products and Litton granulocytes 5516 Nicholson Lane, Kensington, Maryland 20795 Telephone: (301) 881-1557 1979 Litton Bionetics, tnc Get the most out of your high quality cytotoxic antibodies with LOW-TOX-M RABBIT COMPLEMENT LOW TOXICITY HIGH ACTIVITY Presentation: CL 3051 5 x 1 ml, lyophilized $30.00 When it comes to COMPLEMENT... come to CEDARLANE Direct orders or inquiries to: UNITED STATES: WORLDWIDE EXCEPT U.S. ,4 C~L CEDARLANE ACCURATE CHEMICAL & LABORATO RI ES SCIENTIFIC CORPORATION LIMITED 5516-8TH LINE, R.R. 2 28 TEC STREET, HICKSVtLLE, N.Y. 11801 HORNBY, ONTARIO, CANADA LOP 1E0 Telephone -

Stress and the Immune System Tracy B
4 World Health • 47th Yeor, No. 2, Morch-Aprill994 Stress and the immune system Tracy B. Herbert any people have the effects of factors as diverse as experienced the examinations, bereavement, divorce, Mconnection between stress unemployment, mental arithmetic, and getting sick. Colds, influenza, and looking after a relative with herpes and allergies seem worse Alzheimer's di sease. In general, when we are severely stressed at these studies find that stress is work or in the home. Others are related to changes in both the never sick until they go on vacation numbers of white blood cells in (that is, after the stress is over), and circulation and the quantity of then they spend the whole time antibody in the blood. Moreover, fighting the virus. Because of stress is associated with changes in intrinsic connections like these, the functioning of immune cells. many researchers are today That is, there is a relatively large exploring whether (and how) stress decrease in both lymphocyte and illness are actually linked. One proliferation and natural killer cell specific focus of this research is to activity in individuals who have study the effects of stress on the experienced stress. There seems to immune systems; after all, if stress A lymphocyte: stress may weaken the capacity be some connection between the affects immunity, that would be one of lymphocytes to combat infection. duration of the stress and the amount way in which stress could contribute of immune change. For example, the to illness. longer the stress, the greater the The function of the immune proliferation"- by incubating these decrease in the number of specific system is to protect us from cells for several days with types of white blood cells. -

Med-Pathway Zoom Workshop
MCAT Immunology Dr. Phillip Carpenter medpathwaymcat Med-pathway AAMC MCAT Content Outline: Immunology Category 1A: Structure/Function of Proteins/AA Immune System Category 3B: Organ Systems Innate vs. Adaptive Immunity T and B Lymphocytes Macrophages & Phagocytes Tissue-Bone marrow, Spleen, Thymus, Lymph nodes Antigen and Antibody Antigen Presentation Clonal Selection Antigen-Antibody recognition Structure of antibody molecule Self vs. Non-self, Autoimmune Diseases Major Histocompatibility Complex Lab Techniques: ELISA & Western Blotting Hematopoiesis Creates Immune Cells Self vs. Non-self Innate vs Adaptive Innate Immunity Physical Barriers: Skin, mucous membranes, pH Inflammatory mediators: Complement, Cytokines, Prostaglandins Cellular Components: Phagocytes-Neutrophils, Eosinophils, Basophils, Mast Cells Antigen Presenting Cells-Monocytes, Macrophages, Dendritic Cells Adaptive (Acquired) Immunity Composed of B and T lymphocytes: Activated by Innate Immunity B cells: Express B cell receptor and secrete antibodies as plasma cells T cells: Mature in thymus, express TCR surface receptor; Activated by Antigen Presenting Cells (APCs) Direct Immune response (The Ringleaders of immune system) Major Lymphoid Organs TYPE SITE FUNCTION Fetal production of Liver 1° lymphoid cells Hematopoietic production of 1° Bone marrow myeloid and lymphoid cells Receives bone marrow T 1° Thymus cells; site where self is selected from non-self Lymph nodes 2° Sites of antigen activation Spleen of lymphocytes; clearance Macrophages (Sentinel Cells) Pattern Recognition -

Association Between Neutrophil-Lymphocyte Ratio and Herpes Zoster Infection in 1688 Living Donor Liver Transplantation Recipients at a Large Single Center
biomedicines Article Association between Neutrophil-Lymphocyte Ratio and Herpes Zoster Infection in 1688 Living Donor Liver Transplantation Recipients at a Large Single Center Ji-Hoon Sim, Young-Jin Moon, Sung-Hoon Kim, Kyoung-Sun Kim , Ju-Seung Lee, Jun-Gol Song * and Gyu-Sam Hwang Department of Anesthesiology and Pain Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul 05505, Korea; [email protected] (J.-H.S.); [email protected] (Y.-J.M.); [email protected] (S.-H.K.); [email protected] (K.-S.K.); [email protected] (J.-S.L.); [email protected] (G.-S.H.) * Correspondence: [email protected]; Tel.: +82-2-3010-3869 Abstract: Liver transplantation (LT) is closely associated with decreased immune function, a contrib- utor to herpes zoster (HZ). However, risk factors for HZ in living donor LT (LDLT) remain unknown. Neutrophil-lymphocyte ratio (NLR) and immune system function are reportedly correlated. This study investigated the association between NLR and HZ in 1688 patients who underwent LDLT between January 2010 and July 2020 and evaluated risk factors for HZ and postherpetic neuralgia (PHN). The predictive power of NLR was assessed through the concordance index and an integrated discrimination improvement (IDI) analysis. Of the total cohort, 138 (8.2%) had HZ. The incidence of HZ after LT was 11.2 per 1000 person-years and 0.1%, 1.3%, 2.9%, and 13.5% at 1, 3, 5, and 10 years, Citation: Sim, J.-H.; Moon, Y.-J.; Kim, respectively. In the Cox regression analysis, preoperative NLR was significantly associated with HZ S.-H.; Kim, K.-S.; Lee, J.-S.; Song, J.-G.; (adjusted hazard ratio [HR], 1.05; 95% confidence interval [CI], 1.02–1.09; p = 0.005) and PHN (HR, Hwang, G.-S. -

Homeostatic and Activated Conditions Cell
The Journal of Immunology Dynamics of the Splenic Innate-like CD19+CD45Rlo Cell Population from Adult Mice in Homeostatic and Activated Conditions Bele´n de Andre´s,*,1 Carmen Prado,* Beatriz Palacios,* Mario Alı´a,* Sharmili Jagtap,† Natalia Serrano,† Isabel Cortegano,* Miguel Angel R. Marcos,† and Maria Luisa Gaspar*,1 In the adult spleen, CD19+CD45R2/lo (19+45Rlo) lymphocytes of embryonic origin exist as a distinct population to that of the conventional B cell lineage. These cells display a plasmablast phenotype, and they spontaneously secrete IgG1 and IgA, whereas the bone marrow population of 19+45Rlo cells contains B1 progenitors. In this study, we show that 19+45Rlo cells are also present in Peyer’s patches and in the spleen throughout the life span of wild-type mice, beginning at postnatal day 7. Although this population is heterogeneous, the surface phenotype of most of these cells distinguishes them from follicular, transitional, marginal zone, and B1 cells. In CBA/CaHN mice, few 19+45Rlo cells were detected at postnatal day 7, and none was observed in the adult spleen. Splenic 19+45Rlo cells exhibited homeostatic BrdU uptake in vivo and actively transcribed cell cycle genes. When trans- ferred to immunodeficient RAG22/2gchain2/2 recipient mice, 19+45Rlo cells survived and differentiated into IgG1– and IgA– plasma cells. Moreover, in vitro stimulation of splenic 19+45Rlo cells with LPS, CpG, BAFF/IL4, and CD40/IL4 induced cell proliferation, IgG1/IgA secretion and the release of IL-10, suggesting a potential immunoregulatory role for this subset of innate- like B cells. The Journal of Immunology, 2012, 189: 2300–2308. -

Lymphocyte-Activation Gene 3 (LAG-3) Immune Pathway
Lymphocyte-Activation Gene 3 (LAG-3) About LAG-3 LAG-3 Lymphocyte-activation gene 3 (LAG-3) is an immune checkpoint receptor protein found on the cell surface of effector T cells and regulatory T cells (Tregs) and functions to control T cell response, activation and growth.1 TCR T cells are a type of white blood cell that are part of the immune system. Activation of cytotoxic T cells by antigens enables them to 1 kill unhealthy or foreign cells. Inactive T cell Antigen MHC Dendritic cell (APC) LAG-3 and LAG-3 and LAG-3 and Immune Function T Cell Exhaustion Cancer • After a T cell is activated to kill its • However, in certain situations where T • Because of its critical role in regulating target cell, LAG-3 expression is cells experience prolonged exposure to an exhaustion of cytotoxic T cells and Treg increased to turn off the immune antigen, such as cancer or chronic function, LAG-3 has become a target of response, so that the T cell does not go infection, the T cells become desensitized study in the cancer field. on to attack healthy cells.2 and lose their ability to activate and multiply in the presence of the antigen.4 • In cancer, LAG-3 expressing exhausted • Inhibition of the immune response is cytotoxic T cells and Tregs expressing accomplished through activation of • The desensitized T cell will also LAG-3 gather at tumor sites.5,6 the LAG-3 pathway, which can occur progressively fail to produce cytokines via binding of LAG-3 to a type of (proteins that assist in the immune • Preclinical studies suggest that inhibiting antigen-presenting complex called response) and kill the target cells.4 LAG-3 allows T cells to regain their MHC II. -

Understanding the Immune System: How It Works
Understanding the Immune System How It Works U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES NATIONAL INSTITUTES OF HEALTH National Institute of Allergy and Infectious Diseases National Cancer Institute Understanding the Immune System How It Works U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES NATIONAL INSTITUTES OF HEALTH National Institute of Allergy and Infectious Diseases National Cancer Institute NIH Publication No. 03-5423 September 2003 www.niaid.nih.gov www.nci.nih.gov Contents 1 Introduction 2 Self and Nonself 3 The Structure of the Immune System 7 Immune Cells and Their Products 19 Mounting an Immune Response 24 Immunity: Natural and Acquired 28 Disorders of the Immune System 34 Immunology and Transplants 36 Immunity and Cancer 39 The Immune System and the Nervous System 40 Frontiers in Immunology 45 Summary 47 Glossary Introduction he immune system is a network of Tcells, tissues*, and organs that work together to defend the body against attacks by “foreign” invaders. These are primarily microbes (germs)—tiny, infection-causing Bacteria: organisms such as bacteria, viruses, streptococci parasites, and fungi. Because the human body provides an ideal environment for many microbes, they try to break in. It is the immune system’s job to keep them out or, failing that, to seek out and destroy them. Virus: When the immune system hits the wrong herpes virus target or is crippled, however, it can unleash a torrent of diseases, including allergy, arthritis, or AIDS. The immune system is amazingly complex. It can recognize and remember millions of Parasite: different enemies, and it can produce schistosome secretions and cells to match up with and wipe out each one of them. -

A Novel BCMA/CD3 Bispecific T-Cell Engager for the Treatment
OPEN Leukemia (2017) 31, 1743–1751 www.nature.com/leu ORIGINAL ARTICLE A novel BCMA/CD3 bispecific T-cell engager for the treatment of multiple myeloma induces selective lysis in vitro and in vivo S Hipp1, Y-T Tai2,3, D Blanset4, P Deegen5, J Wahl5, O Thomas5, B Rattel5, PJ Adam1, KC Anderson2,3 and M Friedrich5 B-cell maturation antigen (BCMA) is a highly plasma cell-selective protein that is expressed on malignant plasma cells of multiple myeloma (MM) patients and therefore is an ideal target for T-cell redirecting therapies. We developed a bispecific T-cell engager (BiTE) targeting BCMA and CD3ε (BI 836909) and studied its therapeutic impacts on MM. BI 836909 induced selective lysis of BCMA- positive MM cells, activation of T cells, release of cytokines and T-cell proliferation; whereas BCMA-negative cells were not affected. Activity of BI 836909 was not influenced by the presence of bone marrow stromal cells, soluble BCMA or a proliferation-inducing ligand (APRIL). In ex vivo assays, BI 836909 induced potent autologous MM cell lysis in both, newly diagnosed and relapsed/ refractory patient samples. In mouse xenograft studies, BI 836909 induced tumor cell depletion in a subcutaneous NCI-H929 xenograft model and prolonged survival in an orthotopic L-363 xenograft model. In a cynomolgus monkey study, administration of BI 836909 led to depletion of BCMA-positive plasma cells in the bone marrow. Taken together, these results show that BI 836909 is a highly potent and efficacious approach to selectively deplete BCMA-positive MM cells and represents a novel immunotherapeutic for the treatment of MM. -

B-Cell Lymphomas Differ in Their Responsiveness to Cpg Oligodeoxynucleotides
1490 Vol. 11, 1490–1499, February 15, 2005 Clinical Cancer Research B-Cell Lymphomas Differ in their Responsiveness to CpG Oligodeoxynucleotides Bernd Jahrsdorfer,5 Lars Mu¨hlenhoff,1 responsiveness to CpG oligodeoxynucleotides. Focusing Sue E. Blackwell,5 Moritz Wagner,1 clinical studies on patients with highly CpG oligodeoxynu- cleotide–sensitive B-cell malignancies may improve the Hendrik Poeck,1 Evelyn Hartmann,3 Ralf Jox,1 clinical outcome of such trials. Thomas Giese,4 Bertold Emmerich,2 Stefan Endres,1 5 1 George J. Weiner, and Gunther Hartmann INTRODUCTION 1 2 Division of Clinical Pharmacology and Department of Internal The vast majority of lymphoid neoplasms worldwide are Medicine, Division of Hematology, and 3Department of Otorhinolaryngology, University of Munich, Munich, Germany; derived from B lymphocytes at various stages of differentiation. 4Institute of Immunology, University of Heidelberg, Heidelberg, Neoplasms originating from precursor B cells are rare. Neo- Germany; and 5Holden Comprehensive Cancer Center and plasms derived from mature naive B cells include B-cell chronic Department of Internal Medicine, University of Iowa, Iowa City, Iowa lymphocytic leukemia (B-CLL), B-cell small lymphocytic lymphoma (SLL), and mantle cell lymphoma (MCL). Follicular lymphoma (FL) and diffuse large B-cell lymphoma (LCL) are ABSTRACT derived from germinal center B cells. Memory B cells can Human B cells detect CpG motifs within microbial DNA develop into marginal zone B-cell lymphoma (MZL) and B- via TLR9. Synthetic CpG oligodeoxynucleotides are currently CLL. Plasmacytoma is related to plasma cells (1). The most being tested in clinical trials for the therapy of different types typical lymphomas are of diffuse large B-cell type (33%) of B cell non-Hodgkin’s lymphoma. -

Immunology 101
Immunology 101 Justin Kline, M.D. Assistant Professor of Medicine Section of Hematology/Oncology Committee on Immunology University of Chicago Medicine Disclosures • I served as a consultant on Advisory Boards for Merck and Seattle Genetics. • I will discuss non-FDA-approved therapies for cancer 2 Outline • Innate and adaptive immune systems – brief intro • How immune responses against cancer are generated • Cancer antigens in the era of cancer exome sequencing • Dendritic cells • T cells • Cancer immune evasion • Cancer immunotherapies – brief intro 3 The immune system • Evolved to provide protection against invasive pathogens • Consists of a variety of cells and proteins whose purpose is to generate immune responses against micro-organisms • The immune system is “educated” to attack foreign invaders, but at the same time, leave healthy, self-tissues unharmed • The immune system can sometimes recognize and kill cancer cells • 2 main branches • Innate immune system – Initial responders • Adaptive immune system – Tailored attack 4 The immune system – a division of labor Innate immune system • Initial recognition of non-self (i.e. infection, cancer) • Comprised of cells (granulocytes, monocytes, dendritic cells and NK cells) and proteins (complement) • Recognizes non-self via receptors that “see” microbial structures (cell wall components, DNA, RNA) • Pattern recognition receptors (PRRs) • Necessary for priming adaptive immune responses 5 The immune system – a division of labor Adaptive immune system • Provides nearly unlimited diversity of receptors to protect the host from infection • B cells and T cells • Have unique receptors generated during development • B cells produce antibodies which help fight infection • T cells patrol for infected or cancerous cells • Recognize “foreign” or abnormal proteins on the cell surface • 100,000,000 unique T cells are present in all of us • Retains “memory” against infections and in some cases, cancer.