Functional Morphology and Evolution of Stem Succulence in Cacti
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
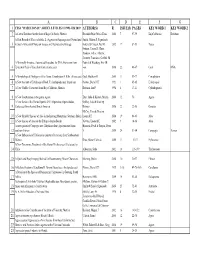
Haseltonia Articles and Authors.Xlsx
ABCDEFG 1 CSSA "HASELTONIA" ARTICLE TITLES #1 1993–#26 2019 AUTHOR(S) R ISSUE(S) PAGES KEY WORD 1 KEY WORD 2 2 A Cactus Database for the State of Baja California, Mexico Resendiz Ruiz, María Elena 2000 7 97-99 BajaCalifornia Database A First Record of Yucca aloifolia L. (Agavaceae/Asparagaceae) Naturalized Smith, Gideon F, Figueiredo, 3 in South Africa with Notes on its uses and Reproductive Biology Estrela & Crouch, Neil R 2012 17 87-93 Yucca Fotinos, Tonya D, Clase, Teodoro, Veloz, Alberto, Jimenez, Francisco, Griffith, M A Minimally Invasive, Automated Procedure for DNA Extraction from Patrick & Wettberg, Eric JB 4 Epidermal Peels of Succulent Cacti (Cactaceae) von 2016 22 46-47 Cacti DNA 5 A Morphological Phylogeny of the Genus Conophytum N.E.Br. (Aizoaceae) Opel, Matthew R 2005 11 53-77 Conophytum 6 A New Account of Echidnopsis Hook. F. (Asclepiadaceae: Stapeliae) Plowes, Darrel CH 1993 1 65-85 Echidnopsis 7 A New Cholla (Cactaceae) from Baja California, Mexico Rebman, Jon P 1998 6 17-21 Cylindropuntia 8 A New Combination in the genus Agave Etter, Julia & Kristen, Martin 2006 12 70 Agave A New Series of the Genus Opuntia Mill. (Opuntieae, Opuntioideae, Oakley, Luis & Kiesling, 9 Cactaceae) from Austral South America Roberto 2016 22 22-30 Opuntia McCoy, Tom & Newton, 10 A New Shrubby Species of Aloe in the Imatong Mountains, Southern Sudan Leonard E 2014 19 64-65 Aloe 11 A New Species of Aloe on the Ethiopia-Sudan Border Newton, Leonard E 2002 9 14-16 Aloe A new species of Ceropegia sect. -

Species Classification and Nomenclature by Norbert Leist and Andrea Jonitz Prof
ISTA Purity Seminar 15. June 2009 Zürich TlTools for seed identifi cati on species classification and nomenclature by Norbert Leist and Andrea Jonitz Prof. Dr. Norbert Leist Dr. Andrea Jonitz Brahmsstr.25 LTZ Augustenberg 76669 Bad Schönborn Neßlerstr.23 Germany 76227 Karlsruhe [email protected] Germany [email protected] Aquilegia vulgaris, Variation Variation • Variation is everywhere in biological systems. Natural variation at the population level is usualy not continuous, but occurs in discrete units or taxa. Easily the most important taxonomic level is the species because it is often the smallest clearly recognizable and discrete set of populations. • Understanding how species form and how to recognize them have been major challenges to systematists. The variation in one population becomes interrupted, the way to a split into two species strong hairy nearly glabrous Variation on species • Sources of variation: MttiMutation Recombination Independent assortment of the chromosomes Random genetic drift Selection Conservation of species characteristics avoiding gene flow Isolating barriers: temporal (seasonal, diurnal) habitat (wet, dry; calceous, silicious) floral (structural, behavioral eg. adaptations for pollinators) reproductive mode (self fertilisation, agamospery) incompatibility (pollen, seeds) hybrid inviability hybrid floral isolation hybrid sterility hybrid break down Iris germanica Iris sibirica Isolation by habitat Definition of „species“ is not easy A species is the smallest aggregation of populations -

Excerpted From
Excerpted from © by the Regents of the University of California. All rights reserved. May not be copied or reused without express written permission of the publisher. click here to BUY THIS BOOK CHAPTER ›3 ‹ ROOT STRUCTURE AND FUNCTION Joseph G. Dubrovsky and Gretchen B. North Introduction Structure Primary Structure Secondary Structure Root Types Development and Growth Indeterminate Root Growth Determinate Root Growth Lateral Root Development Root System Development Adaptations to Deserts and Other Arid Environments Root Distribution in the Soil Environmental Effects on Root Development Developmental Adaptations Water and Mineral Uptake Root Hydraulic Conductivity Mineral Uptake Mycorrhizal and Bacterial Associations Carbon Relations Conclusions and Future Prospects Literature Cited rocky or sandy habitats. The goals of this chapter are to re- Introduction view the literature on the root biology of cacti and to pres- From the first moments of a plant’s life cycle, including ent some recent findings. First, root structure, growth, and germination, roots are essential for water uptake, mineral development are considered, then structural and develop- acquisition, and plant anchorage. These functions are es- mental adaptations to desiccating environments, such as pecially significant for cacti, because both desert species deserts and tropical tree canopies, are analyzed, and finally and epiphytes in the cactus family are faced with limited the functions of roots as organs of water and mineral up- and variable soil resources, strong winds, and frequently take are explored. 41 (Freeman 1969). Occasionally, mucilage cells are found in Structure the primary root (Hamilton 1970).Figure3.1nearhere: Cactus roots are less overtly specialized in structure than Differentiation of primary tissues starts soon after cell are cactus shoots. -

South American Cacti in Time and Space: Studies on the Diversification of the Tribe Cereeae, with Particular Focus on Subtribe Trichocereinae (Cactaceae)
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2013 South American Cacti in time and space: studies on the diversification of the tribe Cereeae, with particular focus on subtribe Trichocereinae (Cactaceae) Lendel, Anita Posted at the Zurich Open Repository and Archive, University of Zurich ZORA URL: https://doi.org/10.5167/uzh-93287 Dissertation Published Version Originally published at: Lendel, Anita. South American Cacti in time and space: studies on the diversification of the tribe Cereeae, with particular focus on subtribe Trichocereinae (Cactaceae). 2013, University of Zurich, Faculty of Science. South American Cacti in Time and Space: Studies on the Diversification of the Tribe Cereeae, with Particular Focus on Subtribe Trichocereinae (Cactaceae) _________________________________________________________________________________ Dissertation zur Erlangung der naturwissenschaftlichen Doktorwürde (Dr.sc.nat.) vorgelegt der Mathematisch-naturwissenschaftlichen Fakultät der Universität Zürich von Anita Lendel aus Kroatien Promotionskomitee: Prof. Dr. H. Peter Linder (Vorsitz) PD. Dr. Reto Nyffeler Prof. Dr. Elena Conti Zürich, 2013 Table of Contents Acknowledgments 1 Introduction 3 Chapter 1. Phylogenetics and taxonomy of the tribe Cereeae s.l., with particular focus 15 on the subtribe Trichocereinae (Cactaceae – Cactoideae) Chapter 2. Floral evolution in the South American tribe Cereeae s.l. (Cactaceae: 53 Cactoideae): Pollination syndromes in a comparative phylogenetic context Chapter 3. Contemporaneous and recent radiations of the world’s major succulent 86 plant lineages Chapter 4. Tackling the molecular dating paradox: underestimated pitfalls and best 121 strategies when fossils are scarce Outlook and Future Research 207 Curriculum Vitae 209 Summary 211 Zusammenfassung 213 Acknowledgments I really believe that no one can go through the process of doing a PhD and come out without being changed at a very profound level. -

THE* RELATIONSHIP of Drosophila Rdgrospiracula AND'erwinia
The relationship of Drosophila nigrospiracula and Ervinia carnegieana to the bacterial necrosis of Carnegiea gigantea Item Type text; Thesis-Reproduction (electronic) Authors Graf, Penelope Ann, 1941- Publisher The University of Arizona. Rights Copyright © is held by the author. Digital access to this material is made possible by the University Libraries, University of Arizona. Further transmission, reproduction or presentation (such as public display or performance) of protected items is prohibited except with permission of the author. Download date 26/09/2021 16:49:18 Link to Item http://hdl.handle.net/10150/318458 THE* RELATIONSHIP OF Drosophila rdgrospiracula AND'Erwinia. carnegieana TO THE BACTERIAL NECROSIS OF Carnegiea gigantea. by Penelope Ann Graf A Thesis Submitted to the Faculty of the DEPARTMENT OF ZOOLOGY In Partial Fulfillment of the Requirements For the Degree of MASTER OF SCIENCE In the Graduate College THE UNIVERSITY OF ARIZONA 19 6 5 STATEMENT BY AUTHOR This thesis has been submitted in partial fulfillment of requirements for an advanced degree at The University of Arizona and is deposited in the University Library to be made available to borrowers under rules of the Library. Brief quotations from this thesis are allowable without special permission, provided that accurate acknowledgment of source is made. Requests for permission for extended quotation from or reproduction of this manuscript in whole or in part may be granted by the head of the major department or the Dean of the Graduate College when in his judgment the proposed use of the material is in the interests of scholarship. In all other instances, however, permission must be obtained from the author. -

S1 the Three Areas of Importance for the Fruticose Ramalinaceae.FINAL
Supplementary Data S1 Three Major Ecogeographic Areas of Evolution in Fruticose Ramalinaceae Three geographical areas appear significant to the evolutionary history of lichens in arid regions. We focus on three fruticose genera of the Ramalinaceae: Nambialina (gen. nov.), Niebla, and Vermilacinia. They have adapted to obtaining moisture from fog in the following coastal regions of our study: (I) California-Baja California coastal chaparral and Vizcaíno deserts (II) South America Atacama and Sechura deserts (III) South Africa coastal fynbos and Namib Desert The botanical significance of each of these will be briefly discussed. Chapter (I) further includes updates on the ecogeographical data and evolutionary interpretation for the genera Niebla and Vermilacinia in Baja California. (I) California and Baja California Coastal Chaparral, Chaparral-Desert Transition and Vizcaíno Deserts 1. California and Baja California Coastal Chaparral The lichen flora of the Baja California peninsula (Mexico, Baja California and Baja California Sur) is pretty well known as a result of the many splendid contributions by lichenologists to the « Lichen Flora of the Greater Sonoran Desert Region » (Nash et al. 2002, 2004, 2007). The « Greater » portion extends the lichen flora to chaparral, oak woodlands, and conifer forests in southern California, northern Baja California and southern Nevada and all of Arizona; to the deserts of Mojave, Arizona, Vizcaíno, and Magdalena; and to the subtropical vegetation in Baja California Sur and Sonora, Mexico that include lowland mixed deciduous-succulent bushlands, open deciduous montane woodlands/forests, and the evergreen pine-oak woodlands/forests. California, a botanically rich and diverse region with many endemic species (Raven and Axelrod 1978 ; Calsbeek et al. -

Redalyc.Fruits, Seeds and Germination in Five Species of Globose Cacteae
Interciencia ISSN: 0378-1844 [email protected] Asociación Interciencia Venezuela Loza Cornejo, Sofía; Terrazas, Teresa; López Mata, Lauro Fruits, seeds and germination in five species of globose Cacteae (Cactaceae) Interciencia, vol. 37, núm. 3, marzo, 2012, pp. 197-203 Asociación Interciencia Caracas, Venezuela Available in: http://www.redalyc.org/articulo.oa?id=33922725006 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative FRUITS, SEEDS AND GERMINATION IN FIVE SPECIES OF GLOBOSE CACTEAE (CACTACEAE) Sofía Loza-Cornejo, Teresa Terrazas and Lauro López-Mata SUMMARY The morphological characteristics of fruits and seeds, and the weight, and fruit width. Larger fruits with more seeds are ob- germination responses of freshly matured seeds of five species of served for F. histrix, whereas smaller fruits with less weight and Cacteae (Coryphantha bumamma, C. clavata, C. cornifera, Fero- fewer seeds are seen for C. clavata. Seed germination is a rapid cactus histrix and Mammillaria uncinata) were studied at room process and usually starts on the third day. High percentages of temperature under laboratory conditions. The aim of the study germination (>80%) are observed on the sixth day in F. histrix was to record the macro- and micro-morphology of fruits and and M. uncinata. It is concluded that some morphological cha- seeds of these species and to investigate specific requirements racteristics of fruits and seeds can be used to support further for germination. Variance analysis detected significant differen- systematic studies of Cactoideae genera and will contribute new ces (p<0.05) for several variables: number of seeds per fruit, knowledge for their potential use and conservation. -

Myxomycetes Associated with Dryland Ecosystems of the Tehuacán-Cuicatlán Valley Biosphere Reserve, Mexico
Fungal Diversity Myxomycetes associated with dryland ecosystems of the Tehuacán-Cuicatlán Valley Biosphere Reserve, Mexico Estrada-Torres, A.1, Wrigley de Basanta, D.2, Conde, E.1 and Lado, C.2* 1Centro de investigación en Ciencias Biológicas, Universidad Autónoma de Tlaxcala, Apartado Postal 183, Tlaxcala 90,000, Tlaxcala, México. 2Departamento de Micología. Real Jardín Botánico, CSIC, Plaza de Murillo, 2. 28014 Madrid. Spain. Estrada-Torres, A., Wrigley de Basanta, D., Conde, E. and Lado, C. (2009). Myxomycetes associated with dryland ecosystems of the Tehuacán-Cuicatlán Valley Biosphere Reserve, Mexico. Fungal Diversity 36: 17-56. The results of a biodiversity survey of a xeric Mexican Biosphere Reserve are presented. This survey represents the first intensive study of cacti and succulent plants ever carried out for myxomycetes. The results include 104 species and one variety, identified from 1200 records from field and moist chamber culture collections. Two new species (Didymium tehuacanense and Perichaena stipitata), found on decayed remains of succulent plants, are described. Eleven species (Comatricha reticulospora, Cribraria lepida, Didymium clavodecus, D. eremophilum, D. orthonemata, D. sturgisii, D. subreticulosporum, Licea belmontiana, Macbrideola oblonga, M. synsporos and Perichaena quadrata) are new records for the Neotropics, and seven others taxa have not been recorded previously from Mexico. Taxonomic comments, data on distribution and SM, LM and SEM micrographs of selected species are included. An analysis of the relationships that exist between myxomycetes and the substrates on which they develop confirms the presence of a distinct assemblage of myxomycetes associated with specific plants from arid environments. Hypotheses are proposed for the patterns of species distribution and the ecological requirements of this specialised myxobiota. -

Abstract Alelopatía Diferencial Entre Los Géneros De
MARCELA AVENDAÑO-GONZÁLEZ1, ERNESTO I. BADANO1,3, JORGE E. RAMÍREZ- ALBORES1, JOEL FLORES1, JORGE A. FLORES-CANO2 Abstract The Peruvian peppertree (Schinus molle) is a dioecious species from South America that was introduced into central Mexico fve centuries ago. This tree has invaded abandoned agricultural felds from semiarid regions, where it can be found with several native succulent plants that have recolonized these areas. Al- though peppertrees have negative allelopathic effects on crops, their effects on these native plants remain unknown. Indeed, the allelopathy of peppertrees has only been tested for female individuals, while the allelopathic potential of male peppertrees has not been assessed yet. This study focused on these issues and assessed whether peppertrees affect germination of succulent plants from the Chihuahuan Desert and whether these effects differ between male and female trees. For this we conducted a series of germina- tion bioassays where seeds of native species were watered with aqueous extracts of staminate fowers and leaves produced by male peppertrees, and with aqueous extracts of fruits and leaves produced by female peppertrees. Additionally, we conducted experiments where seeds of native species were sowed on soils collected beneath the canopy of both tree genders. The results of all these experiments indicated that both peppertree genders can reduce germination of native species, but also suggested that male peppertrees would have stronger allelopathic effects than female peppertrees. To our best knowledge, this is the frst study reporting allelopathic effects of peppertrees on native plants from Mexico, but this is also the frst study indicating differential gender effects for invasive dioecious species with allelopathic potential. -

Posgrado En Ciencias Ambientales Efectos
POSGRADO EN CIENCIAS AMBIENTALES EFECTOS INHIBITORIOS DE LA ESPECIE EXÓTICA SCHINUS MOLLE L. (ANACARDIACEAE) SOBRE LA GERMINACIÓN DE ESPECIES NATIVAS DE MÉXICO Tesis que presenta MARCELA AVENDAÑO GONZÁLEZ Para obtener el grado de Maestra en Ciencias Ambientales Director de tesis Dr. Ernesto Iván Badano San Luis Potosí, S.L.P. Agosto de 2014 I Acta de Examen DEJAR ESTA PÁGINA EN BLANCO IV Agradecimientos Le doy gracias a mis padres, en especial a mi mamá porque sin su esfuerzo, dedicación y apoyo no sería la persona que soy. A mi hermana por siempre apoyarme y acompañarme. A mi familia. De manera muy especial, quiero agradecer a mi director de tesis, el Dr. Ernesto Iván Badano por todas las enseñanzas, consejos y motivaciones. Por sacarme de mi zona de confort, pero principalmente por dedicar tiempo y esfuerzo en mi. Al Dr Joel David Flores Rivas por los consejos, aporte y asesoría brindada para la presente tesis, así como por su disposición amable en todo momento. Al Dr Jorge Alberto Flores Cano por los aportes brindados a la presente tesis. A la División de Ciencias Ambientales del Instituto Potosino de Investigación Científica y Tecnológica, así como a los profesores-investigadores por motivarme a aprender a lo largo del camino. Al CONACYT por el apoyo económico brindado para la realización de la maestría. A los técnicos de la División de Ciencias Ambientales, en especial al M. en C. Juan Pablo Rodas Ortíz por su ayuda, disposición y apoyo para la realización de los experimentos. Finalmente, me gustaría agradecer a mis compañeros de maestría: Esmeralda, Tonatiuh, Alejandra y Daniela, fue un placer haber compartido estos dos años con ustedes. -

The Aboriginal Utilization of the Tall Cacti in the American Southwest
University of New Mexico UNM Digital Repository UNM Bulletins Scholarly Communication - Departments 1937 The ba original utilization of the tall cacti in the American Southwest Edward Franklin Castetter Willis Harvey Bell Follow this and additional works at: https://digitalrepository.unm.edu/unm_bulletin Recommended Citation Castetter, Edward Franklin and Willis Harvey Bell. "The bora iginal utilization of the tall cacti in the American Southwest." University of New Mexico biological series, v. 5, no. 1, University of New Mexico bulletin, whole no. 307, Ethnobiological studies in the American Southwest, 4 5, 1 (1937). https://digitalrepository.unm.edu/unm_bulletin/28 This Article is brought to you for free and open access by the Scholarly Communication - Departments at UNM Digital Repository. It has been accepted for inclusion in UNM Bulletins by an authorized administrator of UNM Digital Repository. For more information, please contact [email protected]. t11111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111III The University of New Mexico Bulletin Ethnobiological Studies in the American Southwest I IV. The Aboriginal Utilization ofthe Tall Cacti in the American Southwest By EDWARD F. CASTETTER, Professor of Biology University of New Mexico and WILLIS H. BELL, Associate Professor of Biology . U '. ....1-. Uni~e?ity of New Mexico :II ~~I-D6. U.,IV/,;it, .&-'-;1. -

The Giant Columnar Cactus Pachycereus Pringlei Adaptively Modifies Its Stem Shape from the Dry Tropics Into the Arid Mid-Latitude Deserts
Journal of Arid Environments xxx (2017) 1e8 Contents lists available at ScienceDirect Journal of Arid Environments journal homepage: www.elsevier.com/locate/jaridenv The giant columnar cactus Pachycereus pringlei adaptively modifies its stem shape from the dry tropics into the arid mid-latitude deserts * Mariana Delgado-Fernandez a, Pedro P. Garcillan a, , Exequiel Ezcurra b a Centro de Investigaciones Biologicas Del Noroeste, Av. Instituto Politecnico Nacional 195, La Paz, Baja California Sur 23096, Mexico b University of California Riverside, 900 University Avenue, Riverside, CA 92521, USA article info abstract Article history: Because of the lack of leaves, the distributions of columnar cacti are limited by their capacity to trade off Received 30 September 2016 their photosynthetic chlorenchyma and their non-photosynthetic storage parenchyma. For species with Received in revised form wide latitudinal ranges, variations in stem surface area:volume ratios could play an adaptive role. Based 13 April 2017 on the fact that Pachycereus pringlei spans more than a 1000 km along the Baja California Peninsula, we Accepted 3 July 2017 used this species to analyze changes in stem allometry between populations. We selected six sites, from Available online xxx latitude 23e31 N, ranging from 518 to 55 mm of annual rainfall. We used an allometric model to analyze the diameter-to-height relationship, estimating the parameters through linear modeling. The Keywords: fi Allometry height of the main stem when the rst branch emerges was estimated by regressing the height of the Branching height plant against the number of lateral shoots. The solar radiation intercepted by an unbranched 6-m-tall Cardon cardon was estimated using an irradiance model.