Canine Tarsus Stabilization Surgical Technique 1 2
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
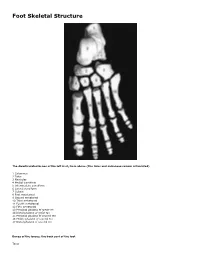
Skeletal Foot Structure
Foot Skeletal Structure The disarticulated bones of the left foot, from above (The talus and calcaneus remain articulated) 1 Calcaneus 2 Talus 3 Navicular 4 Medial cuneiform 5 Intermediate cuneiform 6 Lateral cuneiform 7 Cuboid 8 First metatarsal 9 Second metatarsal 10 Third metatarsal 11 Fourth metatarsal 12 Fifth metatarsal 13 Proximal phalanx of great toe 14 Distal phalanx of great toe 15 Proximal phalanx of second toe 16 Middle phalanx of second toe 17 Distal phalanx of second toe Bones of the tarsus, the back part of the foot Talus Calcaneus Navicular bone Cuboid bone Medial, intermediate and lateral cuneiform bones Bones of the metatarsus, the forepart of the foot First to fifth metatarsal bones (numbered from the medial side) Bones of the toes or digits Phalanges -- a proximal and a distal phalanx for the great toe; proximal, middle and distal phalanges for the second to fifth toes Sesamoid bones Two always present in the tendons of flexor hallucis brevis Origin and meaning of some terms associated with the foot Tibia: Latin for a flute or pipe; the shin bone has a fanciful resemblance to this wind instrument. Fibula: Latin for a pin or skewer; the long thin bone of the leg. Adjective fibular or peroneal, which is from the Greek for pin. Tarsus: Greek for a wicker frame; the basic framework for the back of the foot. Metatarsus: Greek for beyond the tarsus; the forepart of the foot. Talus (astragalus): Latin (Greek) for one of a set of dice; viewed from above the main part of the talus has a rather square appearance. -

Equine MRI, Edited by Rachel C
Chapter 17 The p roximal t arsal r egion Rachel Murray , Natasha Werpy , Fabrice Audigi é , Jean - Marie Denoix , Matthew Brokken and Thorben Schulze INTRODUCTION Imaging of the proximal tarsus can be achieved in both standing and anaes- thetized horses. The height of the horse and the amount of hind end mus- culature determines which magnet confi guration will produce the best images of this region. A horse with short limbs and wide, well - muscled quarters may be more diffi cult to fi t into the long, narrow bore of some high - fi eld magnets under general anaesthesia. In the same way, the side of the lateral recumbency should be considered because it is generally easier to bring the proximal tarsus of the lower hind limb closer to the isocentre of the magnet compared with the upper one. Well developed hind limb musculature can prevent the limb from being placed far enough into the bore to allow the tarsus to be positioned at the isocentre of the magnet. The tarsus of a horse with this body type (or stature) is more easily positioned in a standing system. In contrast, for a narrow horse with long limbs, the height of the tarsus and width between the limbs may make it more diffi cult to image this region within a standing system. The degree of sway in the proximal aspect of the limbs in a standing, sedated horse is often substan- tially greater than in the distal aspect of the limbs. This degree of sway can produce signifi cant motion artefact and can make it frustrating to acquire images of the proximal tarsus. -

The Occurrence of Tarsal Injuries in Male Mice of C57BL/6N Substrains in Multiple
bioRxiv preprint doi: https://doi.org/10.1101/2020.02.25.964254; this version posted February 25, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 Long title: The occurrence of tarsal injuries in male mice of C57BL/6N substrains in multiple 2 international mouse facilities 3 4 Short title: Tarsal injury in C57BL/6N male mice 5 6 Eleanor Herbert2, Michelle Stewart1, Marie Hutchison1, Ann M. Flenniken4,5, Dawei Qu4,5, 7 Lauryl M. J. Nutter4,6, Colin McKerlie4,6, Liane Hobson1, Brenda Kick3, Bonnie Lyons3, Jean- 8 Paul Wiegand3, Rosalinda Doty3, Juan Antonio Aguilar-Pimentel7, Martin Hrabe de Angelis7,8,9, 9 Mary Dickinson10, John Seavitt10, Jacqueline K. White3, Cheryl L Scudamore1, Sara Wells1* 10 11 12 13 Affiliations 14 1 Mary Lyon Centre, MRC Harwell Institute, Harwell Campus, Oxfordshire, OX11 0RD, UK 15 2 Department of Pathobiology and Population Sciences, Royal Veterinary College, Hertfordshire, 16 UK AL9 7TA, UK 17 3 The Jackson Laboratory, 600 Main Street, Bar Harbor, Maine 04609, USA 18 4 The Centre for Phenogenomics, Toronto, ON, Canada, M5T 3H7 19 5 Lunenfeld-Tanenbaum Research Institute, Sinai Health, 600 University Avenue, Toronto, 20 ON, Canada, M5G 1X5 21 6 The Hospital for Sick Children, 555 University Avenue, Toronto, ON, Canada, M5G 1X8 22 7German Mouse Clinic, Institute of Experimental Genetics, Helmholtz Zentrum München, 23 Ingolstädter Landstraße 1, 85764 Neuherberg, Germany 24 8School of Life Science Weihenstephan, Technische Universität München, Alte Akademie 8, 25 85354 Freising, Germany 26 9German Center for Diabetes Research (DZD), Ingolstädter Landstr. -

Radiography and Radiographic Interpretation of the Tarsus (VET-473) Western Veterinary Conference 2004 Timothy R
Radiography and Radiographic Interpretation of the Tarsus (VET-473) Western Veterinary Conference 2004 Timothy R. O'Brien, DVM, PhD, DACVR University of California, Davis Davis, CA, USA 18276161 I. Introduction Tarsal diseases are a common cause of hind limb lameness in the horse. The clinical signs of tarsal disease often resemble those of stifle disease particularly the response to flexion tests. As a result both the stifle and tarsus are commonly radiographed to identify pathologic change in the horse with a hind limb lameness and during purchase evaluations. It seems prudent for purchase evaluations to radiograph these anatomical areas in both limbs. Radiographic interpretation of the tarsus is considered difficult by most equine practitioners. There seems to be three major causes of this difficulty. These causes relate to the numerous bones comprising the tarsus, variety of shapes of the tarsal bones, and veterinarians tend to be less familiar with the radiographic anatomy and pathology of the tarsus than most other anatomical regions, particularly regions of the forelimb. I am going to make some general statements in an attempt to simplify the radiographic interpretation of the tarsus. These general statements are: Most radiographic findings are located at the dorsum of the tarsus. Pathologic abnormalities of the tarsus tend to be in specific anatomical areas. Soft tissue swelling (STS) is a key for assisting to localize the abnormality. There are only a few radiographic diagnoses for the tarsus. The diagnoses of abnormalities of the tarsus identified with radiology can be classified in the following way: 1. Soft tissue swelling without bony change 2. -

Bones of the Hindlimb
BONES OF THE HINDLIMB Andrea Heinzlmann University of Veterinary Medicine Budapest Department of Anatomy and Histology 1st Oktober 2019 BONES OF THE HINDLIMB COMPOSED OF: 1. PELVIC GIRDLE (CINGULUM MEMBRI PELVINI) 2. THIGH 3. LEG (CRUS) 4. FOOT (PES) BONES OF THE PELVIC LIMB (OSSA MEMBRI PELVINI) PELVIC GIRDLE (CINGULUM MEMBRI PELVINI): - connection between the pelvic limb and the trunk consists of: 1. two HIP BONES (OSSA COXARUM) Hip bones of a pig, dorsal aspect Hip bones of a pig, ventral aspect PELVIC GIRDLE (CINGULUM MEMBRI PELVINI) HIP BONE (OS COXAE): - in young animals each hip bone comprises three bones: 1. ILIUM (OS ILII) – craniodorsal 2. PUBIS (OS PUBIS) – cranioventral 3. ISHIUM (OS ISCHI) – caudoventral - all three bones united by a synchondrosis - the synchondrosis ossifies later in life Hip bones of an ox, left lateral aspect Hip bones of an ox, ventrocranial aspect PELVIC GIRDLE (CINGULUM MEMBRI PELVINI) HIP BONE (OS COXAE): ACETABULUM: - ilium, pubis and ischium meet at the acetabulum Left acetabulum of a horse, lateral aspect Hip bones of a dog, right lateral aspect Left acetabulum of an ox, lateral aspect PELVIC GIRDLE (CINGULUM MEMBRI PELVINI) HIP BONE (OS COXAE): SYMPHYSIS PELVINA: - the two hip bones united ventrally at the symphysis pelvina by a fibrocartilaginous joint ossified with advancing age - in females the fibrocartilage of the symphysis becomes loosened during pregnancy by action of hormones Hip bones of a horse, ventrocranial aspect Hip bones of an ox, ventrocranial aspect PELVIC GIRDLE (CINGULUM MEMBRI PELVINI) HIP BONE (OS COXAE): SYMPHYSIS PELVINA: - in females the fibrocartilage of the symphysis becomes loosened during pregnancy by action of hormones http://pchorse.se/index.php/en/articles/topic-of-the-month/topics-topics/4395-mars2017-eng PELVIC GIRDLE (CINGULUM MEMBRI PELVINI) HIP BONE (OS COXAE): SYMPHYSIS PELVINA divided into: 1. -

How to Interpret Radiographs of the Carpus and Tarsus of the Young Performance Horse
HOW TO TAKE AND INTERPRET RADIOGRAPHS OF THE YOUNG PERFORMANCE HORSE How to Interpret Radiographs of the Carpus and Tarsus of the Young Performance Horse Elizabeth M. Santschi, DVM, Diplomate ACVS Author’s address: Department of Veterinary Clinical Sciences, The Ohio State University, 601 Vernon L. Tharp Street, Columbus, OH 43210; [email protected]. © 2013 AAEP. 1. Introduction there are areas that are more commonly affected by Routine radiographic evaluation of young perfor- RA, and they should receive the closest scrutiny. mance horses has become commonplace in Thor- These will be denoted by black circles on the radio- oughbreds but also occurs in other performance graphic images included in these proceedings. RA breeds. This practice has resulted in the frequent best detected on a specific projection will be listed in discovery of radiographic abnormalities (RA) that bold, but should be confirmed on other projections are often clinically silent but can cause concern in when possible. Finally, readers should always con- buyers and sellers of young performance stock. sider three general factors when reviewing radio- The prevalence of radiographic developmental or- graphic images: thopedic disease in 6-month-old horses has been re- (1) Make sure that the films are of the correct ported to be 25% in Warmbloods, 41% in horse. Standardbreds, and 34% in Thoroughbreds.1 In (2) Make sure the date is appropriate and Thoroughbred yearlings intended for racing, 86.3% consistent. exhibit RA2; in young Standardbreds, 42%3; in year- (3) Make sure all required views are present and ling Warmblood horses, 69.5%4; and in 1- and of acceptable quality. -

The Tarsus of Erythrosuid Archosaurs, and Implications for Early Diapsid
Zoological Journal of the Linnean Society (1996), 116: 347–375. With 11 figures The tarsus of erythrosuchid archosaurs, and implications for early diapsid phylogeny DAVID J. GOWER Department of Geology, University of Bristol, Wills Memorial Building, Queens Road, Bristol, BS8 1RJ, UK and Institut und Museum f¨ur Geologie und Pal¨aontologie, Universit¨at T¨ubingen, Sigwartstrasse 10, D-72076 T¨ubingen, Germany Received September 1994, accepted for publication February 1995 The morphology of the erythrosuchid ankle joint is reassessed. Two specimens, recently thought to have been incorrectly referred to Erythrosuchus africanus, are shown without doubt to belong to this taxon. Furthermore, the morphology is essentially similar to that of other early archosaurs. The tarsus of Erythrosuchus is poorly ossified and consists of a calcaneum, astragalus, and two distal tarsals. The calcanea of Erythrosuchus, Vjushkovia triplicostata, and Shansisuchus shansisuchus are all similar in being dorsoventrally compressed, possessing a lateral tuber, and lacking a perforating foramen. The astragalus of V. triplicostata is currently unknown. The astragalus of Shansisuchus is apparently unique in form. The erythrosuchid pes is therefore more derived than has been recently proposed. The tarsal morphology of several other archosauromorph taxa is reviewed and many details are found to be at variance with the literature. The plesiomorphic condition for the Archosauromorpha consists of four distal tarsals and a proximal row of three elements; two of which articulate with the tibia. These proximal elements are interpreted as the astragalus, calcaneum, and a centrale, and the same pattern is retained in the earliest archosaurs. This reassessed tarsal morphology has implications for the homology of the centrale and reconstruction of early diapsid phylogeny. -
Lab Manual Appendicular Skele
1 PRE-LAB EXERCISES When studying the skeletal system, the bones are often sorted into two broad categories: the axial skeleton and the appendicular skeleton. This lab focuses on the appendicular skeleton, which is formed from the pectoral and pelvic girdles and the upper and lower limbs. View Module 7.2 Axial and Appendicular Skeleton to highlight the bones of the appendicular skeleton and compare them to those of the axial skeleton. Examine Module 11.1 Appendicular Skeleton to view only the bones of the appendicular skeleton. In addition to learning about all the bones of the appendicular skeleton, it is also important to identify some significant bone markings. Bone markings can have many shapes, including holes, round or sharp projections, and shallow or deep valleys, among others. These markings on the bones serve many purposes, including forming attachments to other bones or muscles and allowing passage of a blood vessel or nerve. It is helpful to understand the meanings of some of the more common bone marking terms. Before we get started, look up the definitions of these common bone marking terms: Canal: Condyle: Facet: Fissure: Foramen: (see Module 10.18 Foramina of Skull) Fossa: Margin: Process: Proximal: Trochanter: Tubercle: Tuberosity: Throughout this exercise, you will notice bold terms. This is meant to focus your attention on these important words. Make sure you pay attention to any bold words and know how to explain their definitions and/or where they are located. Use the following modules to guide your exploration of the appendicular skeleton. As you explore these bones in Visible Body’s app, also locate the bones and bone markings on any available charts, models, or specimens. -

Insect Morphology - Legs 1
INSECT MORPHOLOGY - LEGS 1 * The fact that arthropods are and have been serially metameric animals from their very early beginnings has provided an evolutionary platform from which great diversity has been achieved. The presence of similar appendages on each of the metameres which were presumably primitively designed for walking has in great part been responsible for the great diversity seen today. * Today the segmental appendages serve many functions. Walking, swimming, jumping, carrying, digging, grasping, feeding, etc. * The most primitive arthropods known, the trilobites had segmental appendages which were fully segmented in available fossils. Note the labrum over the mouth and the 18 pairs of segmental appendages (4+3+11) which are all similar and apparently function for walking. The fossil record does not extend further back to the trilobite ancestors to allow us a glimpse of how arthropod legs arrived at a primitively 8-segmented condition. So, we must turn to embryology and comparative anatomy to gain insight into phylogenetic relationships among the arthropods. We still will gain very little understanding into the phylogenetic development of arthropod limbs. * Embryological evidence shows that regardless of the final adult form, all arthropod appendages have the same origin in the embryo, namely, from paired, lateroventral bud-like lobes of the body segments. Also, the embryonic development of the legs in the arthropods is very similar to that seen in the onychophorans, suggesting that they are homologous. The musculature of the 2 groups also supports their being homologous. * The leg is a tubular outgrowth of the body wall. Its movable sections are called podomeres, and are merely the sclerotized parts of the tube, and the joints are short unsclerotized parts between them. -

The Carpus and Tarsus of the Early Permian Synapsid Sphenacodon Ferox (Eupelycosauria: Sphenacodontidae)
Lucas, S.G. and Zeigler, K.E., eds., 2005, The Nonmarine Permian, New Mexico Museum of Natural History and Science Bulletin No. 30. 106 THE CARPUS AND TARSUS OF THE EARLY PERMIAN SYNAPSID SPHENACODON FEROX (EUPELYCOSAURIA: SPHENACODONTIDAE) AMY C. HENRICI1, DAVID S BERMAN1, SPENCER G. LUCAS2, ANDREW B. HECKERT2, LARRY F. RINEHART2, AND KATE E. ZEIGLER3 1Section of Vertebrate Paleontology, Carnegie Museum of Natural History, 4400 Forbes Avenue, Pittsburgh, PA 15213-4080; 2New Mexico Museum of Natural History & Science, 1801 Mountain Road, NW, Albuquerque, NM 87104; 3Department of Earth and Planetary Sciences, University of New Mexico, Albuquerque, NM 87131 Abstract—The carpus and tarsus of the sphenacodontid synapsid Sphenacodon ferox are described in detail for the first time on the basis of nearly complete, loosely articulated examples from an incomplete, partially articu- lated skeleton from the Lower Permian El Cobre Canyon Formation of the Cutler Group of north-central New Mexico. On the one hand, the carpus and tarsus of S. ferox are compared to those of the more derived Dimetrodon and only member of Sphenacodontidae in which these structures are otherwise known adequately, and, on the other, to Haptodus, the sole member of the sister taxon of Sphenacodontoidea (Sphenacodontidae plus Therapsida). In order to recognize possible morphological trends in the carpus and tarsus within the combined clades of Haptodus and Sphenacodontidae, members of the sister clades Ophiacodontidae and Varanopseidae are utilized for outgroup comparison. INTRODUCTION the Lower Permian of New Mexico (Romer and Price, 1940; Eberth, 1985). The Late Pennsylvanian to Early Permian pelycosaurian-grade Weight of the matrix-encased specimens and the remoteness of or basal synapsid amniotes include seven genera of Sphenacodontidae, the Cardillo Quarry necessitated that the three specimens be removed some of which became a common component of Early Permian assem- as two plaster-jacketed blocks. -

Local Control of Leg Movements and Motor Patterns During Grooming in Locusts
The Journal of Neuroscience, December 15, 1996, 16(24):8067–8078 Local Control of Leg Movements and Motor Patterns during Grooming in Locusts Ari Berkowitz and Gilles Laurent Division of Biology, California Institute of Technology, Pasadena, California 91125 This study demonstrates that the thoracic and abdominal ner- tors, in contrast to hindleg EMGs during walking. During vous system of locusts is sufficient to mediate several site- grooming of the ear, hindleg tibial extension/flexion and tibial specific and distinct grooming leg movements. Locusts can use extensor/flexor muscle bursts can occur independently of the a hindleg or middle leg to groom at least four ipsilateral thoracic femur elevation/depression and trochanteral levator/depressor and abdominal sites, without input from the brain, subesopha- muscle bursts, suggesting that the neural modules controlling geal ganglion, or prothoracic ganglion. The hindleg is used to tibial and femoral movements can be uncoupled during this groom the posterior abdomen, the ventral or posterior hindleg behavior. Tibial extension can occur before, or even in the coxa, and the ear; the middle leg is used to groom the anterior absence of, tibial extensor muscle activity, suggesting that hindleg coxa. Grooming movements are often rhythmic and spring-like properties of the leg and energy transfer from femur display site-specific intralimb coordination patterns. During motion may play important roles in such leg movements. Ad- grooming of the posterior abdomen or ventral hindleg coxa, for jacent legs sometime show coordinated femur movement dur- example, hindleg tibial extension occurs nearly simultaneously ing grooming with one hindleg, suggesting that grooming may with femur elevation, in contrast with locust hindleg movements also involve interlimb coordination. -
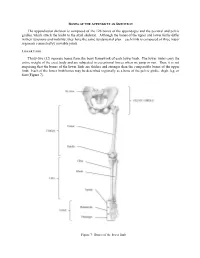
Bones of the Appendicular Skeleton
BONES OF THE APPENDICULAR SKELETON The appendicular skeleton is composed of the 126 bones of the appendages and the pectoral and pelvic girdles, which attach the limbs to the axial skeleton. Although the bones of the upper and lower limbs differ in their functions and mobility, they have the same fundamental plan – each limb is composed of three major segments connected by movable joints. LOWER LIMB Thirty-two (32) separate bones form the bony framework of each lower limb. The lower limbs carry the entire weight of the erect body and are subjected to exceptional forces when we jump or run. Thus, it is not surprising that the bones of the lower limb are thicker and stronger than the comparable bones of the upper limb. Each of the lower limb bones may be described regionally as a bone of the pelvic girdle, thigh, leg, or foot (Figure 7). Figure 7: Bones of the lower limb Pelvic (Hip) Girdle (Marieb / Hoehn – Chapter 7; Pgs. 234 – 238) The pelvic girdle is formed by the paired os coxae (coxal bones). Together with the sacrum and coccyx of the axial skeleton, this group of bones forms the bony pelvis. The ability to bear weight is more important in the pelvic girdle than the pectoral girdle. Thus, the os coxae are heavy and massive with a firm attachment to the axial skeleton. Each os coxa is a result of the fusion of three bones: the ilium, ischium, and pubis. These three bones fuse at the deep hemispherical socket, the acetabulum, which receives the femur. Figure 8: Right os coxa, lateral and medial views A.