The Earliest Roman Counterfeit by Means of Gold/Mercury Amalgam
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
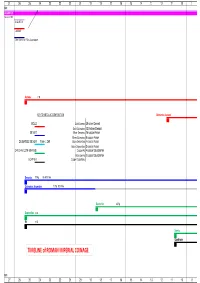
TIMELINE of ROMAN IMPERIAL COINAGE
27 26 25 24 23 22 21 20 19 18 17 16 15 14 13 12 11 10 9 B.C. AUGUSTUS 16 Jan 27 BC AUGUSTUS CAESAR Other title: e.g. Filius Augustorum Aureus 7.8g KEY TO METALLIC COMPOSITION Quinarius Aureus GOLD Gold Aureus 25 silver Denarii Gold Quinarius 12.5 silver Denarii SILVER Silver Denarius 16 copper Asses Silver Quinarius 8 copper Asses DE-BASED SILVER from c. 260 Brass Sestertius 4 copper Asses Brass Dupondius 2 copper Asses ORICHALCUM (BRASS) Copper As 4 copper Quadrantes Brass Semis 2 copper Quadrantes COPPER Copper Quadrans Denarius 3.79g 96-98% fine Quinarius Argenteus 1.73g 92% fine Sestertius 25.5g Dupondius 12.5g As 10.5g Semis Quadrans TIMELINE of ROMAN IMPERIAL COINAGE B.C. 27 26 25 24 23 22 21 20 19 18 17 16 15 14 13 12 11 10 9 8 7 6 5 4 3 2 1 1 2 3 4 5 6 7 8 9 10 11 A.D.A.D. denominational relationships relationships based on Aureus Aureus 7.8g 1 Quinarius Aureus 3.89g 2 Denarius 3.79g 25 50 Sestertius 25.4g 100 Dupondius 12.4g 200 As 10.5g 400 Semis 4.59g 800 Quadrans 3.61g 1600 8 7 6 5 4 3 2 1 1 2 3 4 5 6 7 8 91011 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 19 Aug TIBERIUS TIBERIUS Aureus 7.75g Aureus Quinarius Aureus 3.87g Quinarius Aureus Denarius 3.76g 96-98% fine Denarius Sestertius 27g Sestertius Dupondius 14.5g Dupondius As 10.9g As Semis Quadrans 3.61g Quadrans 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 TIBERIUS CALIGULA CLAUDIUS Aureus 7.75g 7.63g Quinarius Aureus 3.87g 3.85g Denarius 3.76g 96-98% fine 3.75g 98% fine Sestertius 27g 28.7g -

Hadrian and the Greek East
HADRIAN AND THE GREEK EAST: IMPERIAL POLICY AND COMMUNICATION DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of the Ohio State University By Demetrios Kritsotakis, B.A, M.A. * * * * * The Ohio State University 2008 Dissertation Committee: Approved by Professor Fritz Graf, Adviser Professor Tom Hawkins ____________________________ Professor Anthony Kaldellis Adviser Greek and Latin Graduate Program Copyright by Demetrios Kritsotakis 2008 ABSTRACT The Roman Emperor Hadrian pursued a policy of unification of the vast Empire. After his accession, he abandoned the expansionist policy of his predecessor Trajan and focused on securing the frontiers of the empire and on maintaining its stability. Of the utmost importance was the further integration and participation in his program of the peoples of the Greek East, especially of the Greek mainland and Asia Minor. Hadrian now invited them to become active members of the empire. By his lengthy travels and benefactions to the people of the region and by the creation of the Panhellenion, Hadrian attempted to create a second center of the Empire. Rome, in the West, was the first center; now a second one, in the East, would draw together the Greek people on both sides of the Aegean Sea. Thus he could accelerate the unification of the empire by focusing on its two most important elements, Romans and Greeks. Hadrian channeled his intentions in a number of ways, including the use of specific iconographical types on the coinage of his reign and religious language and themes in his interactions with the Greeks. In both cases it becomes evident that the Greeks not only understood his messages, but they also reacted in a positive way. -

The Roman Empire – Roman Coins Lesson 1
Year 4: The Roman Empire – Roman Coins Lesson 1 Duration 2 hours. Date: Planned by Katrina Gray for Two Temple Place, 2014 Main teaching Activities - Differentiation Plenary LO: To investigate who the Romans were and why they came Activities: Mixed Ability Groups. AFL: Who were the Romans? to Britain Cross curricular links: Geography, Numeracy, History Activity 1: AFL: Why did the Romans want to come to Britain? CT to introduce the topic of the Romans and elicit children’s prior Sort timeline flashcards into chronological order CT to refer back to the idea that one of the main reasons for knowledge: invasion was connected to wealth and money. Explain that Q Who were the Romans? After completion, discuss the events as a whole class to ensure over the next few lessons we shall be focusing on Roman Q What do you know about them already? that the children understand the vocabulary and events described money / coins. Q Where do they originate from? * Option to use CT to show children a map, children to locate Rome and Britain. http://www.schoolsliaison.org.uk/kids/preload.htm or RESOURCES Explain that the Romans invaded Britain. http://resources.woodlands-junior.kent.sch.uk/homework/romans.html Q What does the word ‘invade’ mean? for further information about the key dates and events involved in Websites: the Roman invasion. http://www.schoolsliaison.org.uk/kids/preload.htm To understand why they invaded Britain we must examine what http://www.sparklebox.co.uk/topic/past/roman-empire.html was happening in Britain before the invasion. -

Roman Coins Elementary Manual
^1 If5*« ^IP _\i * K -- ' t| Wk '^ ^. 1 Digitized by Google Digitized by Google Digitized by Google Digitized by Google Digitized by Google Digitized by Google PROTAT BROTHERS, PRINTBRS, MACON (PRANCi) Digitized by Google ROMAN COINS ELEMENTARY MANUAL COMPILED BY CAV. FRANCESCO gNECCHI VICE-PRBSIDENT OF THE ITALIAN NUMISMATIC SOaETT, HONORARY MEMBER OF THE LONDON, BELGIAN AND SWISS NUMISMATIC SOCIBTIES. 2"^ EDITION RKVISRD, CORRECTED AND AMPLIFIED Translated by the Rev<> Alfred Watson HANDS MEMBF,R OP THE LONDON NUMISMATIC SOCIETT LONDON SPINK & SON 17 & l8 PICCADILLY W. — I & 2 GRACECHURCH ST. B.C. 1903 (ALL RIGHTS RF^ERVED) Digitized by Google Arc //-/7^. K.^ Digitized by Google ROMAN COINS ELEMENTARY MANUAL AUTHOR S PREFACE TO THE ENGLISH EDITION In the month of July 1898 the Rev. A. W. Hands, with whom I had become acquainted through our common interests and stud- ieSy wrote to me asking whether it would be agreeable to me and reasonable to translate and publish in English my little manual of the Roman Coinage, and most kindly offering to assist me, if my knowledge of the English language was not sufficient. Feeling honoured by the request, and happy indeed to give any assistance I could in rendering this science popular in other coun- tries as well as my own, I suggested that it would he probably less trouble ii he would undertake the translation himselt; and it was with much pleasure and thankfulness that I found this proposal was accepted. It happened that the first edition of my Manual was then nearly exhausted, and by waiting a short time I should be able to offer to the English reader the translation of the second edition, which was being rapidly prepared with additions and improvements. -

GREEK and ROMAN COINS GREEK COINS Technique Ancient Greek
GREEK AND ROMAN COINS GREEK COINS Technique Ancient Greek coins were struck from blank pieces of metal first prepared by heating and casting in molds of suitable size. At the same time, the weight was adjusted. Next, the blanks were stamped with devices which had been prepared on the dies. The lower die, for the obverse, was fixed in the anvil; the reverse was attached to a punch. The metal blank, heated to soften it, was placed on the anvil and struck with the punch, thus producing a design on both sides of the blank. Weights and Values The values of Greek coins were strictly related to the weights of the coins, since the coins were struck in intrinsically valuable metals. All Greek coins were issued according to the particular weight system adopted by the issuing city-state. Each system was based on the weight of the principal coin; the weights of all other coins of that system were multiples or sub-divisions of this major denomination. There were a number of weight standards in use in the Greek world, but the basic unit of weight was the drachm (handful) which was divided into six obols (spits). The drachm, however, varied in weight. At Aigina it weighed over six grammes, at Corinth less than three. In the 6th century B.C. many cities used the standard of the island of Aegina. In the 5th century, however, the Attic standard, based on the Athenian tetradrachm of 17 grammes, prevailed in many areas of Greece, and this was the system adopted in the 4th century by Alexander the Great. -

Roman Imperatorial and Imperial Coinage Bernhard E
Roman Imperatorial and Imperial Coinage Bernhard E. Woytek The Periods • Civil Wars at the End of the Republic (49 – 31 BC) Issues of the Rome Mint – “Imperatorial“ Issues • Principate (31/27 BC – AD 284: Diocletian) “Augustan“ System (with modifications: Caracalla etc.) Imperial Coinage and Provincial Coinages • Dominate (AD 284 – 491: Anastasius I.) Unified Currency. Monetary Reforms of Diocletian, Constantine I. and of Constantine‘s Sons The Roman „Imperial“ Coinage: Three Important Aspects • Creation of a Regular Gold Coinage: Aureus (produced continuously from Caesar onwards), Solidus (created under Constantine I.) • Portrait of the Ruler on the Obverse: Introduced to Rome under Caesar, 44 BC • Creation of the Medallion: Coin-like, non-monetary object, outside the denominational system, but produced by the official mint Gold Coinage Aureus, Julius Caesar, 48 BC Less than 10 pieces known Gold Coinage First Large Issue of Aurei produced under Caesar‘s Rule: Aulus Hirtius, Praetor, 46 BC Die Study (RIN 2003, M. C. Molinari): 537 pieces listed Gold Coinage: Augustus (27 BC – AD 14) 1 Aureus = 25 Denarii (see Cassius Dio 55,12,3) Imperial Bronze Coinage: The Augustan System 1 Denarius = 4 Sestertii From Augustus on in BRASS: Part of the coinage reform, made Senatus Consulto (SC) 1 Sestertius = 2 Dupondii 1 Dupondius = 2 Asses Gold Coinage: Nero (AD 54-68) Nero‘s Monetary Reform (AD 64): Aurei struck on a Standard of 45 to the Roman pound (libra: c. 327g) -> theoretically c. 7.27g (but struck al marco) Gold Coinage: Caracalla (AD 211-217) Introduction of the Double Aureus (Binio) (British Museum, Inv. 1867,0101.772: 13.02g) Gold Coinage: Gallienus (AD 253-268) Aureus, Rome, 264-267 6.07g Aureus, Rome, 257 2.36g „Binio“, Mediolanum, 262 3.64g Weights vary considerably -> coin values had to be established individually Fineness of Imperial Gold Coins Source: C. -

Nummi Serrati, Bigati Et Alii. Coins of the Roman Republic in East-Central Europe North of the Sudetes and the Carpathians
Chapter 2 Outline history of Roman Republican coinage The study of the history of the coinage of the Roman state, including that of the Republican Period, has a history going back several centuries. In the past two hundred years in particular there has been an impressive corpus of relevant literature, such as coin catalogues and a wide range of analytical-descriptive studies. Of these the volume essential in the study of Roman Republican coinage defi nitely continues to be the catalogue provided with an extensive commentary written by Michael H. Crawford over forty years ago.18 Admittedly, some arguments presented therein, such as the dating proposed for particular issues, have met with valid criticism,19 but for the study of Republican coin fi nds from northern territories, substantially removed from the Mediterranean world, this is a minor matter. In the study of coin fi nds recovered in our study area from pre-Roman and Roman period prehistoric contexts, we have very few, and usually quite vague, references to an absolute chronology (i.e. the age determined in years of the Common Era) therefore doubts as to the very precise dating of some of the coin issues are the least of our problems. This is especially the case when these doubts are raised mostly with respect to the older coins of the series. These types seem to have entered the territory to the north of the Sudetes and the Carpathians a few score, and in some cases, even over a hundred, years after their date of minting. It is quite important on the other hand to be consistent in our use of the chronological fi ndings related to the Roman Republican coinage, in our case, those of M. -

The Empire Strikes: the Growth of Roman Infrastructural Minting Power, 60 B.C
The Empire Strikes: The Growth of Roman Infrastructural Minting Power, 60 B.C. – A.D. 68 A dissertation submitted to the Graduate School of the University of Cincinnati in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Department of Classics of the College of Arts and Sciences by David Schwei M.A., University of Cincinnati, December 2012 B.A., Emory University, May 2009 Committee Chairs: Peter van Minnen, Ph.D Barbara Burrell, Ph.D. ABSTRACT Coins permeated the Roman Empire, and they offer a unique perspective into the ability of the Roman state to implement its decisions in Italy and the provinces. This dissertation examines how this ability changed and grew over time, between 60 B.C. and A.D. 68, as seen through coin production. Earlier scholars assumed that the mint at Rome always produced coinage for the entire empire, or they have focused on a sudden change under Augustus. Recent advances in catalogs, documentation of coin hoards, and metallurgical analyses allow a fuller picture to be painted. This dissertation integrates the previously overlooked coinages of Asia Minor, Syria, and Egypt with the denarius of the Latin West. In order to measure the development of the Roman state’s infrastructural power, this dissertation combines the anthropological ideal types of hegemonic and territorial empires with the numismatic method of detecting coordinated activity at multiple mints. The Roman state exercised its power over various regions to different extents, and it used its power differently over time. During the Republic, the Roman state had low infrastructural minting capacity. -

DVRNACOS. Helmeted Head of Roma Right. Rev: AVSCRO
1 WESTERN EUROPE. Southern Gaul. Allobrages (Circa 61-43 BC). Quinarius. Obv: DVRNACOS. Helmeted head of Roma right. Rev: AVSCRO. Warrior, holding spear, on horse galloping right. Depeyrot, NC I, 109. Condition: Near very fine. Weight: 1.87 g. Diameter: 15 mm. Starting price: 40 EUR Estimate: 50 EUR 2 WESTERN EUROPE. Northeast Gaul. Ambiani (Circa 50 BC). Potin Unit. Obv: Stylized horse prancing right; pellets-in-annulets around. Rev: Stylized boar advancing right; pellets-in-annulets around. LT 8460; D&T 449. Condition: Near very fine. Weight: 2.81 g. Diameter: 13 mm. Starting price: 40 EUR Estimate: 50 EUR 3 WESTERN EUROPE. Northwest Gaul. Coriosolites (1st century BC). BI Stater. Obv: Head right. Rev: Warrior riding human-headed horse right; lyre below. LT 6703; D&T 2332. Condition: Very fine, collector's numbers on obverse and reverse. Weight: 6.65 g. Diameter: 23 mm. Starting price: 100 EUR Estimate: 125 EUR 4 WESTERN EUROPE. Northwest Gaul. Coriosolites (Circa 100-50 BC). BI Stater. Obv: Stylized head right. Rev: Stylized biga and driver right; below, boar right. D&T 2340; LT 6598. Condition: Very fine. Weight: 6.50 g. Diameter: 22 mm. Starting price: 80 EUR Estimate: 100 EUR 5 WESTERN EUROPE. Southern Gaul. Volcae-Arecomici (Circa 2nd century BC). Obol. Obv: Stylized head left. Rev: Long cross, with decoration in angles. Cf. Depeyrot, NC I, 263. Condition: Extremely fine. Weight: 0.40 g. Diameter: 8 mm. Starting price: 80 EUR Estimate: 100 EUR 6 CENTRAL EUROPE. Boii. GOLD 1/24 Stater (2nd-1st centuries BC). "Athena Alkis" type. -

Ancient Coin Reference Guide
Ancient Coin Reference Guide Part One Compiled by Ron Rutkowsky When I first began collecting ancient coins I started to put together a guide which would help me to identify them and to learn more about their history. Over the years this has developed into several notebooks filled with what I felt would be useful information. My plan now is to make all this information available to other collectors of ancient coinage. I cannot claim any credit for this information; it has all come from many sources including the internet. Throughout this reference I use the old era terms of BC (Before Christ) and AD (Anno Domni, year of our Lord) rather than the more politically correct BCE (Before the Christian era) and CE (Christian era). Rome With most collections, there must be a starting point. Mine was with Roman coinage. The history of Rome is a subject that we all learned about in school. From Julius Caesar, Marc Anthony, to Constantine the Great and the fall of the empire in the late 5th century AD. Rome first came into being around the year 753 BC, when it was ruled under noble families that descended from the Etruscans. During those early days, it was ruled by kings. Later the Republic ruled by a Senate headed by a Consul whose term of office was one year replaced the kingdom. The Senate lasted until Julius Caesar took over as a dictator in 47 BC and was murdered on March 15, 44 BC. I will skip over the years until 27 BC when Octavian (Augustus) ended the Republic and the Roman Empire was formed making him the first emperor. -

The Solidus – the Dollar of the Middle Ages
The Solidus – the Dollar of the Middle Ages It was the symbol of imperial power in Byzantium. Popular and willingly accepted everywhere in the then-known world, it was admired and copied by many kings in many kingdoms. There was no coin that could be compared to it: the Byzantine solidus. For more than 700 years struck in the same weight and fineness, the solidus was the principal trade coin from Europe throughout Asia – the dollar of the Middle Ages. 1 von 15 www.sunflower.ch Roman Empire, Constantine I the Great (307-337), Solidus, 314, Treverorum Denomination: Solidus Mint Authority: Emperor Constantine I Mint: Treverorum (Trier) Year of Issue: 314 Weight (g): 4.45 Diameter (mm): 24.0 Material: Gold Owner: Sunflower Foundation Constantine the Great had the solidus minted for the first time in the mint of Treverorum (Trier), shortly after his ascension to power. Subsequently the coin was issued in other mints of Constantine's sphere of influence as well. After the emperor had become sole ruler in 324, he made the solidus the standard gold coin of his entire realm. It replaced the aureus, which had been minted since the 3rd century BC, but had lost its reputation in the course of the 3rd century AD. Under most of Constantine's successors, solidi were minted in good quality regarding their fineness and weight. This was why the coins soon became renowned for their solidity. The solidus and its third, the tremissis, were issued until the collapse of the Western Roman Empire, and subsequently by several Germanic tribes of the migration period. -

Daily Life in Ancient Rome
IMMEDIATE RELEASE: October 16, 2006 CONTACT: Jay Beeton or Andy Dickes Telephone: 719-482-9864 email: [email protected] Daily Life in Ancient Rome Does higher education mean a higher salary? Modern-day statistics certainly suggest so, but for ancient Romans looking for a big pay day, it was better to shun the life of a well-read lecturer and instead pursue a career as a Roman Centurion soldier. Throughout history’s most famous and infamous empire, money was used to pay salaries, purchase slaves and glorify emperors. Visitors to the American Numismatic Association Money Museum can learn about life in ancient Rome through its coinage at the new exhibit, “The Die Is Cast: Money of the Ancient World,” which opens Nov. 9. Not surprisingly, the phrase “All Roads Lead to Rome” is derived from the city’s place as the cultural, military and economic center of the ancient world. The economy was based on agriculture, mining, trade and slave labor. The health of the economy was chiefly the responsibility of the emperor, who issued coinage, set wages for soldiers and had the power to cancel debts. Taxes were as sure a thing for Roman citizens as they are today. Imperial taxes were paid in Roman coins and grain, and were used to pay the army, maintain the Imperial household and bureaucracy, and subsidize the city of Rome, from grain for the poor and entertainment to imperial palaces and magnificent public buildings. Like much of today’s currency, ancient Romans could expect to find the likeness of political leaders on their money.