Intraspecific, Interspecific, and Interseries Cross-Compatibility in Lilac
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Department of Planning and Zoning
Department of Planning and Zoning Subject: Howard County Landscape Manual Updates: Recommended Street Tree List (Appendix B) and Recommended Plant List (Appendix C) - Effective July 1, 2010 To: DLD Review Staff Homebuilders Committee From: Kent Sheubrooks, Acting Chief Division of Land Development Date: July 1, 2010 Purpose: The purpose of this policy memorandum is to update the Recommended Plant Lists presently contained in the Landscape Manual. The plant lists were created for the first edition of the Manual in 1993 before information was available about invasive qualities of certain recommended plants contained in those lists (Norway Maple, Bradford Pear, etc.). Additionally, diseases and pests have made some other plants undesirable (Ash, Austrian Pine, etc.). The Howard County General Plan 2000 and subsequent environmental and community planning publications such as the Route 1 and Route 40 Manuals and the Green Neighborhood Design Guidelines have promoted the desirability of using native plants in landscape plantings. Therefore, this policy seeks to update the Recommended Plant Lists by identifying invasive plant species and disease or pest ridden plants for their removal and prohibition from further planting in Howard County and to add other available native plants which have desirable characteristics for street tree or general landscape use for inclusion on the Recommended Plant Lists. Please note that a comprehensive review of the street tree and landscape tree lists were conducted for the purpose of this update, however, only -
Djvu Document
BULL BQT. ~URV.INDIA Vol. 10, NOS.3 & 4 : pp. 397-400, 1968 TAXONOMIC POSITION OF THE GENUS NYCTANTHES B. C. Kmu AND ANIMADE Bosc Znstifulc, Calcutta ABSTRACT The genus Nyctanthes with only one species N. arbor-tristis Linn. having flowers like that of JaJrninm was originally induded in Oleaceae. In view of its strongly quadrangular stem and its apparent resemblance to Tectona and other members of the family Verbenaceae, Airy Shaw (1952) placed the genus in a new subfamily under the family Verbenaceae. Stant (K952) gave some anatomical evidence for the inclusion of Nyctanthes in the Verbenaceae. On the basis of com- parative study of Nyctanthas, along with some members of Oleaceae, Verbenaceae and Loganiaceae on cytology, general anatomy of stem and leaf, wood anatomy, floral anatomy and palynology and also on the preliminary data of the chemical constituents present in the plants, the authors state that Nyctanfhes has not much affinityto the members of the Verbenaceae, although it has some similarity with several oleaceous members, After taking all points into consideration this genus is assigned to a new family Nyctanthaceae. INTRODUCTION thes is consistent with its being incluiled in the The Genus Nyctanthes Linn. with only .one Verbenaceae. On the basis of anatomical and species N. arbor-tristis Linn. was originally included palynological studies on Nyctanthes arbor-tristis, in the family Oleaceae mainly on account of the Kundu (1966) was of opinion that Nyctamthes structure of the flower which is somewhat like that belongs neither to the Oleaceae nor to the .Verben- of Jasminum. A second species N. -

'Ivory Silk' Japanese Tree Lilac
Fact Sheet ST-611 October 1994 Syringa reticulata ‘Ivory Silk’ ‘Ivory Silk’ Japanese Tree Lilac1 Edward F. Gilman and Dennis G. Watson2 INTRODUCTION Although a Lilac, this member of the species is quite different in appearance than those with which gardeners are more familiar (Fig. 1). Its upright habit varies from symmetrical to irregular but is more consistent than the species. Cultivars including ‘Ivory Silk’ and ‘Summer Snow’ could be used instead of the species due to the more consistent habit and more flowers. ‘Ivory Silk’ grows well only in USDA hardiness zones 3 through six (perhaps into 7) and has an oval or pyramidal form when young but spreads to a rounded shape as it grows older. This is a very large shrub or small tree, reaching a height of about 20 to 30 feet with a 15-foot-spread. The huge clusters of creamy white flowers, borne in early summer for about two weeks, are the main ornamental feature but lack the fragrance of the spring-blooming Lilacs -- this Lilac’s fragrance is more suggestive of Privet. GENERAL INFORMATION Scientific name: Syringa reticulata ‘Ivory Silk’ Pronunciation: sih-RING-guh reh-tick-yoo-LAY-tuh Common name(s): ‘Ivory Silk’ Japanese Tree Lilac Family: Oleaceae Figure 1. Mature ‘Ivory Silk’ Japanese Tree Lilac. USDA hardiness zones: 3A through 7A (Fig. 2) Origin: not native to North America sidewalk cutout (tree pit); residential street tree; tree Uses: container or above-ground planter; large has been successfully grown in urban areas where air parking lot islands (> 200 square feet in size); wide pollution, poor drainage, compacted soil, and/or tree lawns (>6 feet wide); medium-sized tree lawns drought are common (4-6 feet wide); recommended for buffer strips around Availability: somewhat available, may have to go out parking lots or for median strip plantings in the of the region to find the tree highway; near a deck or patio; screen; trainable as a standard; narrow tree lawns (3-4 feet wide); specimen; 1. -

Outline of Angiosperm Phylogeny
Outline of angiosperm phylogeny: orders, families, and representative genera with emphasis on Oregon native plants Priscilla Spears December 2013 The following listing gives an introduction to the phylogenetic classification of the flowering plants that has emerged in recent decades, and which is based on nucleic acid sequences as well as morphological and developmental data. This listing emphasizes temperate families of the Northern Hemisphere and is meant as an overview with examples of Oregon native plants. It includes many exotic genera that are grown in Oregon as ornamentals plus other plants of interest worldwide. The genera that are Oregon natives are printed in a blue font. Genera that are exotics are shown in black, however genera in blue may also contain non-native species. Names separated by a slash are alternatives or else the nomenclature is in flux. When several genera have the same common name, the names are separated by commas. The order of the family names is from the linear listing of families in the APG III report. For further information, see the references on the last page. Basal Angiosperms (ANITA grade) Amborellales Amborellaceae, sole family, the earliest branch of flowering plants, a shrub native to New Caledonia – Amborella Nymphaeales Hydatellaceae – aquatics from Australasia, previously classified as a grass Cabombaceae (water shield – Brasenia, fanwort – Cabomba) Nymphaeaceae (water lilies – Nymphaea; pond lilies – Nuphar) Austrobaileyales Schisandraceae (wild sarsaparilla, star vine – Schisandra; Japanese -

Classificatie Van Planten – Nieuwe Inzichten En Gevolgen Voor De Praktijk
B268_dendro_bin 03-11-2009 10:32 Pagina 4 Classificatie van planten – nieuwe inzichten en gevolgen voor de praktijk Ir. M.H.A. Hoffman Er zijn ongeveer 300.000 verschillende (hogere) plantensoorten, die onderling veel of weinig op elkaar lijken. Vroeger werden alle planten- soorten afzonderlijk benaamd, en was niet duidelijk hoe deze soorten ver- want waren. Tegenwoordig worden soorten conform het systeem van Lin- naeus ingedeeld in geslachten. Soorten die sterk verwant zijn aan elkaar hebben dezelfde geslachtsnaam. Geslachten worden op hun beurt weer ingedeeld in families en die op hun beurt weer in ordes, enzovoort. Op deze manier kent het plantenrijk een hiërarchisch indelingsysteem, met verwantschap als basis. Sterke verwantschap is meestal ook zicht- baar aan de uiterlijke gelijkenis. Soorten die veel op elkaar lijken en verwant zijn zitten in dezelfde groep.Verre verwanten zitten ver uit elkaar in het systeem. Om een plant goed te kunnen benamen, moet dus eerst uitgezocht worden aan welke andere soorten deze verwant is. Tot voor kort werd de gelijkenis van planten zen. Deze nieuwe ontwikkeling is van grote voornamelijk bepaald aan uiterlijke kenmerken invloed op de taxonomie en op het classificatie- van bijvoorbeeld bloem en blad. De afgelopen systeem van het plantenrijk. Dit heeft bijvoor- decennia kwamen daar al aanvullende criteria beeld invloed op de familie-indeling en de plaats zoals houtanatomie, pollenmorfologie, chemi- van de familie in het systeem. Maar ook op sche inhoudsstoffen en chromosoomaantallen geslachts- en soortniveau zijn er de nodige ver- bij. De afgelopen jaren hebben DNA-technie- schuivingen. Dit artikel gaat in op de ontstaans- ken een grote vlucht genomen. -

Olea Europaea L. a Botanical Contribution to Culture
American-Eurasian J. Agric. & Environ. Sci., 2 (4): 382-387, 2007 ISSN 1818-6769 © IDOSI Publications, 2007 Olea europaea L. A Botanical Contribution to Culture Sophia Rhizopoulou National and Kapodistrian University of Athens, Department of Biology, Section of Botany, Panepistimioupoli, Athens 157 84, Greece Abstract: One of the oldest known cultivated plant species is Olea europaea L., the olive tree. The wild olive tree is an evergreen, long-lived species, wide-spread as a native plant in the Mediterranean province. This sacred tree of the goddess Athena is intimately linked with the civilizations which developed around the shores of the Mediterranean and makes a starting point for mythological and symbolic forms, as well as for tradition, cultivation, diet, health and culture. In modern times, the olive has spread widely over the world. Key words: Olea • etymology • origin • cultivation • culture INTRODUCTION Table 1: Classification of Olea ewopaea Superdivi&on Speimatophyta-seed plants Olea europaea L. (Fig. 1 & Table 1) belongs to a Division Magnohophyta-flowenng plants genus of about 20-25 species in the family Oleaceae [1-3] Class Magn olio psi da- dicotyledons and it is one of the earliest cultivated plants. The olive Subclass Astendae- tree is an evergreen, slow-growing species, tolerant to Order Scrophulanale- drought stress and extremely long-lived, with a life Family Oleaceae- olive family expectancy of about 500 years. It is indicative that Genus Olea- olive Species Olea europaea L. -olive Theophrastus, 24 centuries ago, wrote: 'Perhaps we may say that the longest-lived tree is that which in all ways, is able to persist, as does the olive by its trunk, by its power of developing sidegrowth and by the fact its roots are so hard to destroy' [4, book IV. -

Syringa Xchinensis
Syringa xchinensis - Chinese Lilac or Rouen Lilac (Oleaceae) ------------------------------------------------------------------------------------------------------------ Syringa x chinensis is a shrub with showy, early the terminal floral buds, flowering in early- and mid- May, very fragrant inflorescences, but Chinese Lilac May and lasting for 1-2 weeks is susceptible to powdery mildew on its foliage by -inflorescences occur mostly as single-flowering late summer. forms, with relatively few cultivars as compared to Common Lilac FEATURES -while deadheading will slightly improve the overall Form vigor and appearance of the shrub, it is usually -medium-sized to large- impractical to perform except on young shrubs sized ornamental shrub Fruits maturing at about 10' tall x -winter persistent, brown dehiscent capsules occur on 10' wide, but sometimes woody fruiting stalks, not ornamentally effective but larger a good identification feature of the genus -upright oval growth habit Twigs in youth, becoming leggy -light brown to brown-gray, lightly lenticeled, with and spreading with age fairly stout stems having moderately-sized floral buds -medium growth rate (often in pairs at the terminus of the season's growth) Culture and smaller vegetative buds (as lateral buds on the -full sun to partial shade flowering stems, or as lateral and terminal buds on -best performance occurs in full sun in moist, well- portions of the shrub that are more shaded) drained, neutral to slightly acidic soils of average Trunk fertility, in areas with good air circulation; it is highly -multi-trunked, light brown, and slightly exfoliating adaptable to poor soils, soils of various pH, drought, in thin strips with maturity, becoming somewhat and pollution, but declines under the heat and high leggy with age, and with basal suckers having a rapid humidity of the Southern U.S. -
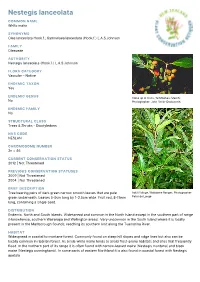
Nestegis Lanceolata
Nestegis lanceolata COMMON NAME White maire SYNONYMS Olea lanceolata Hook.f.; Gymnelaea lanceolata (Hook.f.) L.A.S.Johnson FAMILY Oleaceae AUTHORITY Nestegis lanceolata (Hook.f.) L.A.S.Johnson FLORA CATEGORY Vascular – Native ENDEMIC TAXON Yes ENDEMIC GENUS Close up of fruits, Te Moehau (March). No Photographer: John Smith-Dodsworth ENDEMIC FAMILY No STRUCTURAL CLASS Trees & Shrubs - Dicotyledons NVS CODE NESLAN CHROMOSOME NUMBER 2n = 46 CURRENT CONSERVATION STATUS 2012 | Not Threatened PREVIOUS CONSERVATION STATUSES 2009 | Not Threatened 2004 | Not Threatened BRIEF DESCRIPTION Tree bearing pairs of dark green narrow smooth leaves that are pale Adult foliage, Waitakere Ranges. Photographer: green underneath. Leaves 5-9cm long by 1-2.5cm wide. Fruit red, 8-11mm Peter de Lange long, containing a single seed. DISTRIBUTION Endemic. North and South Islands. Widespread and common in the North Island except in the southern part of range (Horowhenua, southern Wairarapa and Wellington areas). Very uncommon in the South Island where it is locally present in the Marlborough Sounds, reaching its southern limit along the Tuamarina River. HABITAT Widespread in coastal to montane forest. Commonly found on steep hill slopes and ridge lines but also can be locally common in riparian forest. As a rule white maire tends to avoid frost-prone habitats and sites that frequently flood. In the northern part of its range it is often found with narrow-leaved maire (Nestegis montana) and black maire (Nestegis cunninghamii). In some parts of eastern Northland it is also found in coastal forest with Nestegis apetala. FEATURES Stout gynodioecious spreading tree up to 20 m tall usually forming a domed canopy; trunk up to c. -

Syringa Meyeri
Syringa meyeri - Meyer Lilac (Oleaceae) ------------------------------------------------------------------- Syringa meyeri is a compact but spreading, small- Fruits foliaged Lilac with showy, late May, lavender-purple -brown capsules on the winter persistent fruiting inflorescences. Meyer Lilac is especially urban stalks are not ornamental tolerant and without powdery mildew on its foliage. Twigs It is a Lilac that can be grown as a formal or informal -light brown to gray, with winter floral buds that are hedge. small, oval, and distinctly checkered (due to the differential color pattern on the overlapping floral FEATURES bud scales) Form -exhibiting dense twiggy branching on relatively thin -medium-sized ornamental shrub (or small branches (unlike all other Lilacs, which have ornamental tree, when grafted onto a moderate to sparse branching on medium to thick standard) branches) -species form slowly matures at Trunk about 6' tall x 8' wide -usually not applicable, unless the shrub has been -spreading oval growth habit (where grafted onto a standard (typically at about 4' in the oval shape is on its side) height) and becomes tree form -slow growth rate Culture USAGE -full sun to partial shade Function -performs best in full sun in moist, well-drained soils, -foundation, entranceway, border, group planting, but is urban tolerant and adaptable to poor soils, dry informal or formal hedge, or specimen shrub soils, compacted soils, soils of various pH, and Texture especially to heat and drought (but not adaptable to -medium-fine texture in -

Late Lilac Syringa Villosa Plant Guide
Plant Guide LATE LILAC Weediness This plant may become weedy or invasive in some Syringa villosa Vahl regions or habitats and may displace desirable vegetation Plant Symbol = SYVI3 if not properly managed. It does not sucker extensively and its fruit is not desired by birds so the degree of spread Contributed by: USDA NRCS Plant Materials Center, is generally not a problem. Please consult with your local Bismarck, North Dakota NRCS Field Office, Cooperative Extension Service Office, state natural resource, or state agriculture department regarding its status and use. Weed information is also available from the PLANTS Web site at http://plants.usda.gov/. Please consult the Related Web Sites on the Plant Profile for this species for further information. Description Late lilac is native to northern China and is a medium to large, hardy shrub with stout spreading branches. It has an oval to irregularly shaped crown. Flowers are white, or rose to pale lavender. It generally flowers 1- 2 weeks “later” than common lilac, and the color fades quickly (Eisel, 1997). Spreading branches sprout from the base. Plants of this species were 10 feet tall and 12 feet wide Photo Credit: Lincoln-Oakes Nursery, Bismarck, North Dakota after 14 years on loam soils in Conservation Tree and Shrub Group 3 in central South Dakota (Knudson, 2004). Alternate Names This species will coppice back after a light fire or Common Alternate Names: Villous lilac mowing. It is long-lived. Scientific Alternate Names: None The brown buds are opposite. Buds are ⅛ to ½ inch long. Uses The entire leaves are simple and broad-elliptic to oblong. -

Studies on the Trichomes of Some Oleaceae, Structure and Ontogeny
STUDIES ON THE TRICHOMES OF SOME OLEACEAE, STRUCTURE AND ONTOGENY B~ J. A. INAMDAR (Department of Botany, Sardar Patel Univeraity, Vallabh Vidyanagar, Gujarat) Received August 22, 1967 (Communicated by Prof. V. Puff, r.A.sc.) ABSTRACT Some twelve types of tricho~aes are described for four species of Jasminum and Nyctanthes arbor-tristis L. They fall under two main categories, the eglandular and the glandular trichomes. The systematic position of Nyctanthes seems to be quite natural in the family Oleaceae. INTRODUCTION The family Oleaceae has received considerable attention of the botanists particularly with reference to the systcmatic position of Nyctanthes and the monotypic genus Dimetra. Airy SEaw (1952) transferred these gcnera to the Verbenaceae on morphological grounds. Stant (1952) has supported Airy Shaw on anatomical grounds. Johnson (1957) in a review of the family Oleaceae opined that Nyctanthes should be excluded from Oleaceae based on the cytological findings of Taylor (1945). On the other hand the embryo- logical data (Patel, 1963; Kapil, 1967) suggests that Nyctanthes should be retained in the Oleaceae. Several workers (De Bary, 1884; Cowan, 1950, Goodspeed, 1954; Carlquist, 1958, 1959a, 1959b, 1959c, 1961; Mathur, 1961 ; Ramayya, 1962 a, 1962b, 1963) have described the structure and development of trichomes and glands in several angisoperm families. The present investi- gation was carried out to find if the trichomes ~nd glands could be of any help in settling the doubtful systematic position of Oleaceous genera. The present paper deals with the study of eglandular and glandular (glands) trichomes on the vegetative organs of four species of Jasminum, viz., J. -

Diseases of Trees in the Great Plains
United States Department of Agriculture Diseases of Trees in the Great Plains Forest Rocky Mountain General Technical Service Research Station Report RMRS-GTR-335 November 2016 Bergdahl, Aaron D.; Hill, Alison, tech. coords. 2016. Diseases of trees in the Great Plains. Gen. Tech. Rep. RMRS-GTR-335. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 229 p. Abstract Hosts, distribution, symptoms and signs, disease cycle, and management strategies are described for 84 hardwood and 32 conifer diseases in 56 chapters. Color illustrations are provided to aid in accurate diagnosis. A glossary of technical terms and indexes to hosts and pathogens also are included. Keywords: Tree diseases, forest pathology, Great Plains, forest and tree health, windbreaks. Cover photos by: James A. Walla (top left), Laurie J. Stepanek (top right), David Leatherman (middle left), Aaron D. Bergdahl (middle right), James T. Blodgett (bottom left) and Laurie J. Stepanek (bottom right). To learn more about RMRS publications or search our online titles: www.fs.fed.us/rm/publications www.treesearch.fs.fed.us/ Background This technical report provides a guide to assist arborists, landowners, woody plant pest management specialists, foresters, and plant pathologists in the diagnosis and control of tree diseases encountered in the Great Plains. It contains 56 chapters on tree diseases prepared by 27 authors, and emphasizes disease situations as observed in the 10 states of the Great Plains: Colorado, Kansas, Montana, Nebraska, New Mexico, North Dakota, Oklahoma, South Dakota, Texas, and Wyoming. The need for an updated tree disease guide for the Great Plains has been recog- nized for some time and an account of the history of this publication is provided here.