Djvu Document
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Outline of Angiosperm Phylogeny
Outline of angiosperm phylogeny: orders, families, and representative genera with emphasis on Oregon native plants Priscilla Spears December 2013 The following listing gives an introduction to the phylogenetic classification of the flowering plants that has emerged in recent decades, and which is based on nucleic acid sequences as well as morphological and developmental data. This listing emphasizes temperate families of the Northern Hemisphere and is meant as an overview with examples of Oregon native plants. It includes many exotic genera that are grown in Oregon as ornamentals plus other plants of interest worldwide. The genera that are Oregon natives are printed in a blue font. Genera that are exotics are shown in black, however genera in blue may also contain non-native species. Names separated by a slash are alternatives or else the nomenclature is in flux. When several genera have the same common name, the names are separated by commas. The order of the family names is from the linear listing of families in the APG III report. For further information, see the references on the last page. Basal Angiosperms (ANITA grade) Amborellales Amborellaceae, sole family, the earliest branch of flowering plants, a shrub native to New Caledonia – Amborella Nymphaeales Hydatellaceae – aquatics from Australasia, previously classified as a grass Cabombaceae (water shield – Brasenia, fanwort – Cabomba) Nymphaeaceae (water lilies – Nymphaea; pond lilies – Nuphar) Austrobaileyales Schisandraceae (wild sarsaparilla, star vine – Schisandra; Japanese -

Olea Europaea L. a Botanical Contribution to Culture
American-Eurasian J. Agric. & Environ. Sci., 2 (4): 382-387, 2007 ISSN 1818-6769 © IDOSI Publications, 2007 Olea europaea L. A Botanical Contribution to Culture Sophia Rhizopoulou National and Kapodistrian University of Athens, Department of Biology, Section of Botany, Panepistimioupoli, Athens 157 84, Greece Abstract: One of the oldest known cultivated plant species is Olea europaea L., the olive tree. The wild olive tree is an evergreen, long-lived species, wide-spread as a native plant in the Mediterranean province. This sacred tree of the goddess Athena is intimately linked with the civilizations which developed around the shores of the Mediterranean and makes a starting point for mythological and symbolic forms, as well as for tradition, cultivation, diet, health and culture. In modern times, the olive has spread widely over the world. Key words: Olea • etymology • origin • cultivation • culture INTRODUCTION Table 1: Classification of Olea ewopaea Superdivi&on Speimatophyta-seed plants Olea europaea L. (Fig. 1 & Table 1) belongs to a Division Magnohophyta-flowenng plants genus of about 20-25 species in the family Oleaceae [1-3] Class Magn olio psi da- dicotyledons and it is one of the earliest cultivated plants. The olive Subclass Astendae- tree is an evergreen, slow-growing species, tolerant to Order Scrophulanale- drought stress and extremely long-lived, with a life Family Oleaceae- olive family expectancy of about 500 years. It is indicative that Genus Olea- olive Species Olea europaea L. -olive Theophrastus, 24 centuries ago, wrote: 'Perhaps we may say that the longest-lived tree is that which in all ways, is able to persist, as does the olive by its trunk, by its power of developing sidegrowth and by the fact its roots are so hard to destroy' [4, book IV. -

Syringa Xchinensis
Syringa xchinensis - Chinese Lilac or Rouen Lilac (Oleaceae) ------------------------------------------------------------------------------------------------------------ Syringa x chinensis is a shrub with showy, early the terminal floral buds, flowering in early- and mid- May, very fragrant inflorescences, but Chinese Lilac May and lasting for 1-2 weeks is susceptible to powdery mildew on its foliage by -inflorescences occur mostly as single-flowering late summer. forms, with relatively few cultivars as compared to Common Lilac FEATURES -while deadheading will slightly improve the overall Form vigor and appearance of the shrub, it is usually -medium-sized to large- impractical to perform except on young shrubs sized ornamental shrub Fruits maturing at about 10' tall x -winter persistent, brown dehiscent capsules occur on 10' wide, but sometimes woody fruiting stalks, not ornamentally effective but larger a good identification feature of the genus -upright oval growth habit Twigs in youth, becoming leggy -light brown to brown-gray, lightly lenticeled, with and spreading with age fairly stout stems having moderately-sized floral buds -medium growth rate (often in pairs at the terminus of the season's growth) Culture and smaller vegetative buds (as lateral buds on the -full sun to partial shade flowering stems, or as lateral and terminal buds on -best performance occurs in full sun in moist, well- portions of the shrub that are more shaded) drained, neutral to slightly acidic soils of average Trunk fertility, in areas with good air circulation; it is highly -multi-trunked, light brown, and slightly exfoliating adaptable to poor soils, soils of various pH, drought, in thin strips with maturity, becoming somewhat and pollution, but declines under the heat and high leggy with age, and with basal suckers having a rapid humidity of the Southern U.S. -
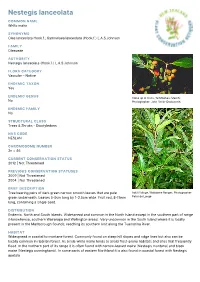
Nestegis Lanceolata
Nestegis lanceolata COMMON NAME White maire SYNONYMS Olea lanceolata Hook.f.; Gymnelaea lanceolata (Hook.f.) L.A.S.Johnson FAMILY Oleaceae AUTHORITY Nestegis lanceolata (Hook.f.) L.A.S.Johnson FLORA CATEGORY Vascular – Native ENDEMIC TAXON Yes ENDEMIC GENUS Close up of fruits, Te Moehau (March). No Photographer: John Smith-Dodsworth ENDEMIC FAMILY No STRUCTURAL CLASS Trees & Shrubs - Dicotyledons NVS CODE NESLAN CHROMOSOME NUMBER 2n = 46 CURRENT CONSERVATION STATUS 2012 | Not Threatened PREVIOUS CONSERVATION STATUSES 2009 | Not Threatened 2004 | Not Threatened BRIEF DESCRIPTION Tree bearing pairs of dark green narrow smooth leaves that are pale Adult foliage, Waitakere Ranges. Photographer: green underneath. Leaves 5-9cm long by 1-2.5cm wide. Fruit red, 8-11mm Peter de Lange long, containing a single seed. DISTRIBUTION Endemic. North and South Islands. Widespread and common in the North Island except in the southern part of range (Horowhenua, southern Wairarapa and Wellington areas). Very uncommon in the South Island where it is locally present in the Marlborough Sounds, reaching its southern limit along the Tuamarina River. HABITAT Widespread in coastal to montane forest. Commonly found on steep hill slopes and ridge lines but also can be locally common in riparian forest. As a rule white maire tends to avoid frost-prone habitats and sites that frequently flood. In the northern part of its range it is often found with narrow-leaved maire (Nestegis montana) and black maire (Nestegis cunninghamii). In some parts of eastern Northland it is also found in coastal forest with Nestegis apetala. FEATURES Stout gynodioecious spreading tree up to 20 m tall usually forming a domed canopy; trunk up to c. -

Studies on the Trichomes of Some Oleaceae, Structure and Ontogeny
STUDIES ON THE TRICHOMES OF SOME OLEACEAE, STRUCTURE AND ONTOGENY B~ J. A. INAMDAR (Department of Botany, Sardar Patel Univeraity, Vallabh Vidyanagar, Gujarat) Received August 22, 1967 (Communicated by Prof. V. Puff, r.A.sc.) ABSTRACT Some twelve types of tricho~aes are described for four species of Jasminum and Nyctanthes arbor-tristis L. They fall under two main categories, the eglandular and the glandular trichomes. The systematic position of Nyctanthes seems to be quite natural in the family Oleaceae. INTRODUCTION The family Oleaceae has received considerable attention of the botanists particularly with reference to the systcmatic position of Nyctanthes and the monotypic genus Dimetra. Airy SEaw (1952) transferred these gcnera to the Verbenaceae on morphological grounds. Stant (1952) has supported Airy Shaw on anatomical grounds. Johnson (1957) in a review of the family Oleaceae opined that Nyctanthes should be excluded from Oleaceae based on the cytological findings of Taylor (1945). On the other hand the embryo- logical data (Patel, 1963; Kapil, 1967) suggests that Nyctanthes should be retained in the Oleaceae. Several workers (De Bary, 1884; Cowan, 1950, Goodspeed, 1954; Carlquist, 1958, 1959a, 1959b, 1959c, 1961; Mathur, 1961 ; Ramayya, 1962 a, 1962b, 1963) have described the structure and development of trichomes and glands in several angisoperm families. The present investi- gation was carried out to find if the trichomes ~nd glands could be of any help in settling the doubtful systematic position of Oleaceous genera. The present paper deals with the study of eglandular and glandular (glands) trichomes on the vegetative organs of four species of Jasminum, viz., J. -

Intraspecific, Interspecific, and Interseries Cross-Compatibility in Lilac
J. AMER.SOC.HORT.SCI. 142(4):279–288. 2017. doi: 10.21273/JASHS04155-17 Intraspecific, Interspecific, and Interseries Cross-compatibility in Lilac Jason D. Lattier 1 and Ryan N. Contreras 2 Department of Horticulture, 4017 Agriculture and Life Sciences Building, Oregon State University, Corvallis, OR 97331-7304 ADDITIONAL INDEX WORDS. Syringa, Pubescentes, Villosae, in vitro germination, controlled crosses, wide hybridization ABSTRACT. Lilacs (Syringa sp.) are a group of ornamental trees and shrubs in the Oleaceae composed of 22–30 species from two centers of diversity: the highlands of East Asia and the Balkan-Carpathian region of Europe. There are six series within the genus Syringa: Pubescentes, Villosae, Ligustrae, Ligustrina, Pinnatifoliae, and Syringa. Intraspecific and interspecific hybridization are proven methods for cultivar development. However, reports of interseries hybridization are rare and limited to crosses among taxa in series Syringa and Pinnatifoliae. Although hundreds of lilac cultivars have been introduced, fertility and cross-compatibility have yet to be formally investigated. Over 3 years, a cross-compatibility study was performed using cultivars and species of shrub-form lilacs in series Syringa, Pubescentes, and Villosae. A total of 114 combinations were performed at an average of 243 ± 27 flowers pollinated per combination. For each combination, we recorded the number of inflorescences and flowers pollinated as well as number of capsules, seed, seedlings germinated, and albino seedlings. Fruit and seed were produced from interseries crosses, but no seedlings were recovered. A total of 2177 viable seedlings were recovered from interspecific and intraspecific combinations in series Syringa, Pubescentes, and Villosae. Albino progeny were produced only from crosses with Syringa pubescens ssp. -

Syringa Reticulata
Syringa reticulata - Japanese Tree Lilac (Oleaceae) --------------------------------------------------------------------------------------- Syringa reticulata is a tree form Lilac with showy, like the branches of Oriental Cherry (Prunus early June, creamy-white inflorescences. Japanese serrulata) Tree Lilac is properly used as a specimen, -stems are constantly forking in a dichotomous entranceway, or street tree without powdery mildew pattern, usually topped by twin terminal buds at the on its foliage. end of the growing season -floral buds are slightly larger than vegetative buds FEATURES Trunk Form -tree form may be either multi-trunked, or single- -medium-sized ornamental tree trunked and limbed up, while the shrub form is multi- or very large ornamental shrub trunked and branching widely at its base -maturing at about 25' tall x 20' -mature trunks are gray, very cherry-like, remaining wide, although larger under smooth for a long time with horizontal lenticels, then optimum conditions eventually transitioning to bark with plates and -upright oval growth habit, fissures becoming more rounded with age USAGE -medium growth rate Function -shrub form may be utilized in borders, rows, group Culture plantings, or as deciduous screens -full sun to partial sun -tree form is found at entranceways, spacious -best performance occurs in full sun in a moist, well- foundations, large raised planters, as a lawn drained soil of average fertility, but it is highly specimen, or as a street tree adaptable to poor soils, compacted soils, various soil -

Flora of South Australia 5Th Edition | Edited by Jürgen Kellermann
Flora of South Australia 5th Edition | Edited by Jürgen Kellermann KEY TO FAMILIES1 J.P. Jessop2 The sequence of families used in this Flora follows closely the one adopted by the Australian Plant Census (www.anbg.gov. au/chah/apc), which in turn is based on that of the Angiosperm Phylogeny Group (APG III 2009) and Mabberley’s Plant Book (Mabberley 2008). It differs from previous editions of the Flora, which were mainly based on the classification system of Engler & Gilg (1919). A list of all families recognised in this Flora is printed in the inside cover pages with families already published highlighted in bold. The up-take of this new system by the State Herbarium of South Australia is still in progress and the S.A. Census database (www.flora.sa.gov.au/census.shtml) still uses the old classification of families. The Australian Plant Census web-site presents comparison tables of the old and new systems on family and genus level. A good overview of all families can be found in Heywood et al. (2007) and Stevens (2001–), although these authors accept a slightly different family classification. A number of names with which people using this key may be familiar but are not employed in the system used in this work have been included for convenience and are enclosed on quotation marks. 1. Plants reproducing by spores and not producing flowers (“Ferns and lycopods”) 2. Aerial shoots either dichotomously branched, with scale leaves and 3-lobed sporophores or plants with fronds consisting of a simple or divided sterile blade and a simple or branched spikelike sporophore .................................................................................. -

Insects Associated with Fruits of the Oleaceae (Asteridae, Lamiales) in Kenya, with Special Reference to the Tephritidae (Diptera)
D. Elmo Hardy Memorial Volume. Contributions to the Systematics and 135 Evolution of Diptera. Edited by N.L. Evenhuis & K.Y. Kaneshiro. Bishop Museum Bulletin in Entomology 12: 135–164 (2004). Insects associated with fruits of the Oleaceae (Asteridae, Lamiales) in Kenya, with special reference to the Tephritidae (Diptera) ROBERT S. COPELAND Department of Entomology, Texas A&M University, College Station, Texas 77843 USA, and International Centre of Insect Physiology and Ecology, Box 30772, Nairobi, Kenya; email: [email protected] IAN M. WHITE Department of Entomology, The Natural History Museum, Cromwell Road, London, SW7 5BD, UK; e-mail: [email protected] MILLICENT OKUMU, PERIS MACHERA International Centre of Insect Physiology and Ecology, Box 30772, Nairobi, Kenya. ROBERT A. WHARTON Department of Entomology, Texas A&M University, College Station, Texas 77843 USA; e-mail: [email protected] Abstract Collections of fruits from indigenous species of Oleaceae were made in Kenya between 1999 and 2003. Members of the four Kenyan genera were sampled in coastal and highland forest habitats, and at altitudes from sea level to 2979 m. Schrebera alata, whose fruit is a woody capsule, produced Lepidoptera only, as did the fleshy fruits of Jasminum species. Tephritid fruit flies were reared only from fruits of the oleaceous subtribe Oleinae, including Olea and Chionanthus. Four tephritid species were reared from Olea. The olive fly, Bactrocera oleae, was found exclusively in fruits of O. europaea ssp. cuspidata, a close relative of the commercial olive, Olea europaea ssp. europaea. Olive fly was reared from 90% (n = 21) of samples of this species, on both sides of the Rift Valley and at elevations to 2801 m. -

Wood Anatomy of the Oleaceae
IAWA Bulletin n.s., Vol. 9 (2),1988,103-182 WOOD ANATOMY OF THE OLEACEAE by Pieter Baas*, Petra M. Esser*, Marijke E. T. van der Westen*, and Marinus Zandee** Contents Summary ...................................................... 104 Introduction . .. 104 Materials and methods ............................................. 106 Phenetic analysis .............................................. 106 Cladistic analysis . 106 Survey of wood anatomical character states in the Oleaceae . .. 107 Introduction 107 - Growth rings 108 - Vessel grouping and distribution 108 Vessel frequency and element size 110 - Vessel perforations 110 - Vessel wall pit- ting 111- Vessel wall thickness and sculpturing 112 - Tyloses and vessel contents 112 - Vascular tracheids 113 - Fibres 113 - Axial parenchyma 127 - Ray tissue 128 - Crystals 129 Generic wood anatomical descriptions . 129 Explanatory note 129 - Abeliophyllwn 129 - Chionanthus (including Linociera) 130 Comoranthus 132-Fontanesia 133-Forestiera 134-Forsythia 135-Fraxinus 135 - Haenianthus 137 - Hesperelaea 137 - Jasminum 138 - Ligustrum 140 Menodora 141-Myxopyrum 142 - Nestegis 142 - Noronhia 143 - Notelaea 143 Nyctanthes 145 - Olea 146 - Osmanthus 149 - Phillyrea 151 - Picconia 152 Schrebera 153 - Syringa 153 - Tessarandra 155 Classification of the Oleaceae . .. 156 Phenetic wood anatomical classification . .. 156 Phylogenetic classification ........................................ 159 The position of Nyctanthes . 166 The wider affinities of the Oleaceae . 167 Ecological and functional considerations. .. 168 A tentative evolutionary scenario for the Oleaceae ........................... 173 Needs for further studies . .. 174 Keys to the woods of the Oleaceae .... .. 174 Comprehensive wood anatomical key to the genera of the Oleaceae . 175 Simplified wood anatomical key to the genera of the Oleaceae. 176 Acknowledgements . .. 177 References . 177 * Rijksherbarium, P.O. Box 9514, 2300 RA Leiden, The Netherlands. ** Institute of Theoretical Biology, Groenhovenstraat 5, 2311 BT Leiden, The Netherlands. 104 IAWA Bulletin n.s., Vol. -

Identification and Control of Invasive Privets (Ligustrum Spp.) in the Middle Southern United States
Invasive Plant Science and Management 2010 3:482–488 Notes and Commentary Identification and Control of Invasive Privets (Ligustrum spp.) in the Middle Southern United States Victor Maddox, John Byrd, Jr., and Brett Serviss* The identification of privet in the middle southern United States can be difficult. Because most introduced species of privet can be invasive, and recent mapping projects seek location and species population data, proper identification is important. Without proper identification of privet species, data on species distributions and other pertinent information regarding invasiveness could lead to improper conclusions. Currently, information on privet identification is scattered throughout a number of reference materials. The purpose of this publication is to assist with the proper identification of escaped privet species, and suggest management options. Nomenclature: Fosamine ammonium; glyphosate; hexazinone; imazapyr; metsulfuron; triclopyr; 2,4-D; 2,4-DP; Amur privet, Ligustrum obtusifolium Sieb. & Zucc. var. suave (Kitagawa) Kitagawa (Syn. L. amurense Carrie`re); border privet, Ligustrum obtusifolium Sieb. & Zucc. var. obtusifolium; California privet, Ligustrum ovalifolium Hassk.; Chinese privet, Ligustrum sinense Lour.; common privet, Ligustrum vulgare L.; glossy privet, Ligustrum lucidum Ait.; Japanese privet, Ligustrum japonicum Thunb.; waxyleaf privet, Ligustrum quihoui Carrie`re. Key words: Invasive species, management. Since the 1700s, at least nine species of privets have been Thunb.) is native to Korea and Japan. The most common introduced into the United States; it is probable that all species in the southern portion of the middle southern were introduced as ornamentals. They have been very region is Chinese privet, although Amu, border, California, successful as ornamentals and continue to be marketed for common or European, glossy, Japanese, and waxyleaf or such purposes. -

Obtuse-Leaved Privet, Border Privet Ligustrum Obtusifolium Siebold & Zucc
Obtuse-leaved privet, Border privet Ligustrum obtusifolium Siebold & Zucc. Olive Family (Oleaceae) DESCRIPTION Obtuse-leaved privet is a deciduous shrub that is multi- stemmed from the base, with spreading, twiggy, branches. It that has become extensively naturalized in disturbed woods and stream valleys. Several other species, Amur privet (L. amurense), California privet (L. ovalifolium), and common or European privet (L. vulgare) are also present as naturalized species in Pennsylvania; however, obtuse-leaved privet is the most common and invasive of these in our area. fruiting stem Height - This shrub grows 10–12 feet tall and 8–10 feet wide. Stem - Branches are slender, light gray, and exhibit an opposite arrangement along the stem. Young stems are hairy. Leaves - Leaves are opposite, firm, smooth, and 1–2 inches long, tapering slightly to a blunt tip and base. Summer leaf color is dark green, becoming purplish in the fall. Flowers - The small white flowers are in clusters at the ends of the branches; they are tubular with 4 spreading petal lobes, and about ⅓ inch long. Blooming occurs in June. Fruit and seed - The small (⅛ inch), black fruits are produced in clusters at the ends of branches; they mature in September and often persist on the plant all winter. Roots - Sprouting can occur from the roots when the tops are cut back severely. flowering stem DISTRIBUTION AND HABITAT Obtuse-leaved privet is native to Japan; it was introduced as a landscape plant in 1860. It has subsequently become naturalized from New Hampshire to North Carolina and west to Michigan, Indiana, and Tennessee. It is present throughout the southern half of Pennsylvania, and at scattered sites elsewhere in the state.