Obtuse-Leaved Privet, Border Privet Ligustrum Obtusifolium Siebold & Zucc
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Djvu Document
BULL BQT. ~URV.INDIA Vol. 10, NOS.3 & 4 : pp. 397-400, 1968 TAXONOMIC POSITION OF THE GENUS NYCTANTHES B. C. Kmu AND ANIMADE Bosc Znstifulc, Calcutta ABSTRACT The genus Nyctanthes with only one species N. arbor-tristis Linn. having flowers like that of JaJrninm was originally induded in Oleaceae. In view of its strongly quadrangular stem and its apparent resemblance to Tectona and other members of the family Verbenaceae, Airy Shaw (1952) placed the genus in a new subfamily under the family Verbenaceae. Stant (K952) gave some anatomical evidence for the inclusion of Nyctanthes in the Verbenaceae. On the basis of com- parative study of Nyctanthas, along with some members of Oleaceae, Verbenaceae and Loganiaceae on cytology, general anatomy of stem and leaf, wood anatomy, floral anatomy and palynology and also on the preliminary data of the chemical constituents present in the plants, the authors state that Nyctanfhes has not much affinityto the members of the Verbenaceae, although it has some similarity with several oleaceous members, After taking all points into consideration this genus is assigned to a new family Nyctanthaceae. INTRODUCTION thes is consistent with its being incluiled in the The Genus Nyctanthes Linn. with only .one Verbenaceae. On the basis of anatomical and species N. arbor-tristis Linn. was originally included palynological studies on Nyctanthes arbor-tristis, in the family Oleaceae mainly on account of the Kundu (1966) was of opinion that Nyctamthes structure of the flower which is somewhat like that belongs neither to the Oleaceae nor to the .Verben- of Jasminum. A second species N. -

'Ivory Silk' Japanese Tree Lilac
Fact Sheet ST-611 October 1994 Syringa reticulata ‘Ivory Silk’ ‘Ivory Silk’ Japanese Tree Lilac1 Edward F. Gilman and Dennis G. Watson2 INTRODUCTION Although a Lilac, this member of the species is quite different in appearance than those with which gardeners are more familiar (Fig. 1). Its upright habit varies from symmetrical to irregular but is more consistent than the species. Cultivars including ‘Ivory Silk’ and ‘Summer Snow’ could be used instead of the species due to the more consistent habit and more flowers. ‘Ivory Silk’ grows well only in USDA hardiness zones 3 through six (perhaps into 7) and has an oval or pyramidal form when young but spreads to a rounded shape as it grows older. This is a very large shrub or small tree, reaching a height of about 20 to 30 feet with a 15-foot-spread. The huge clusters of creamy white flowers, borne in early summer for about two weeks, are the main ornamental feature but lack the fragrance of the spring-blooming Lilacs -- this Lilac’s fragrance is more suggestive of Privet. GENERAL INFORMATION Scientific name: Syringa reticulata ‘Ivory Silk’ Pronunciation: sih-RING-guh reh-tick-yoo-LAY-tuh Common name(s): ‘Ivory Silk’ Japanese Tree Lilac Family: Oleaceae Figure 1. Mature ‘Ivory Silk’ Japanese Tree Lilac. USDA hardiness zones: 3A through 7A (Fig. 2) Origin: not native to North America sidewalk cutout (tree pit); residential street tree; tree Uses: container or above-ground planter; large has been successfully grown in urban areas where air parking lot islands (> 200 square feet in size); wide pollution, poor drainage, compacted soil, and/or tree lawns (>6 feet wide); medium-sized tree lawns drought are common (4-6 feet wide); recommended for buffer strips around Availability: somewhat available, may have to go out parking lots or for median strip plantings in the of the region to find the tree highway; near a deck or patio; screen; trainable as a standard; narrow tree lawns (3-4 feet wide); specimen; 1. -

Outline of Angiosperm Phylogeny
Outline of angiosperm phylogeny: orders, families, and representative genera with emphasis on Oregon native plants Priscilla Spears December 2013 The following listing gives an introduction to the phylogenetic classification of the flowering plants that has emerged in recent decades, and which is based on nucleic acid sequences as well as morphological and developmental data. This listing emphasizes temperate families of the Northern Hemisphere and is meant as an overview with examples of Oregon native plants. It includes many exotic genera that are grown in Oregon as ornamentals plus other plants of interest worldwide. The genera that are Oregon natives are printed in a blue font. Genera that are exotics are shown in black, however genera in blue may also contain non-native species. Names separated by a slash are alternatives or else the nomenclature is in flux. When several genera have the same common name, the names are separated by commas. The order of the family names is from the linear listing of families in the APG III report. For further information, see the references on the last page. Basal Angiosperms (ANITA grade) Amborellales Amborellaceae, sole family, the earliest branch of flowering plants, a shrub native to New Caledonia – Amborella Nymphaeales Hydatellaceae – aquatics from Australasia, previously classified as a grass Cabombaceae (water shield – Brasenia, fanwort – Cabomba) Nymphaeaceae (water lilies – Nymphaea; pond lilies – Nuphar) Austrobaileyales Schisandraceae (wild sarsaparilla, star vine – Schisandra; Japanese -

Olea Europaea L. a Botanical Contribution to Culture
American-Eurasian J. Agric. & Environ. Sci., 2 (4): 382-387, 2007 ISSN 1818-6769 © IDOSI Publications, 2007 Olea europaea L. A Botanical Contribution to Culture Sophia Rhizopoulou National and Kapodistrian University of Athens, Department of Biology, Section of Botany, Panepistimioupoli, Athens 157 84, Greece Abstract: One of the oldest known cultivated plant species is Olea europaea L., the olive tree. The wild olive tree is an evergreen, long-lived species, wide-spread as a native plant in the Mediterranean province. This sacred tree of the goddess Athena is intimately linked with the civilizations which developed around the shores of the Mediterranean and makes a starting point for mythological and symbolic forms, as well as for tradition, cultivation, diet, health and culture. In modern times, the olive has spread widely over the world. Key words: Olea • etymology • origin • cultivation • culture INTRODUCTION Table 1: Classification of Olea ewopaea Superdivi&on Speimatophyta-seed plants Olea europaea L. (Fig. 1 & Table 1) belongs to a Division Magnohophyta-flowenng plants genus of about 20-25 species in the family Oleaceae [1-3] Class Magn olio psi da- dicotyledons and it is one of the earliest cultivated plants. The olive Subclass Astendae- tree is an evergreen, slow-growing species, tolerant to Order Scrophulanale- drought stress and extremely long-lived, with a life Family Oleaceae- olive family expectancy of about 500 years. It is indicative that Genus Olea- olive Species Olea europaea L. -olive Theophrastus, 24 centuries ago, wrote: 'Perhaps we may say that the longest-lived tree is that which in all ways, is able to persist, as does the olive by its trunk, by its power of developing sidegrowth and by the fact its roots are so hard to destroy' [4, book IV. -

Syringa Xchinensis
Syringa xchinensis - Chinese Lilac or Rouen Lilac (Oleaceae) ------------------------------------------------------------------------------------------------------------ Syringa x chinensis is a shrub with showy, early the terminal floral buds, flowering in early- and mid- May, very fragrant inflorescences, but Chinese Lilac May and lasting for 1-2 weeks is susceptible to powdery mildew on its foliage by -inflorescences occur mostly as single-flowering late summer. forms, with relatively few cultivars as compared to Common Lilac FEATURES -while deadheading will slightly improve the overall Form vigor and appearance of the shrub, it is usually -medium-sized to large- impractical to perform except on young shrubs sized ornamental shrub Fruits maturing at about 10' tall x -winter persistent, brown dehiscent capsules occur on 10' wide, but sometimes woody fruiting stalks, not ornamentally effective but larger a good identification feature of the genus -upright oval growth habit Twigs in youth, becoming leggy -light brown to brown-gray, lightly lenticeled, with and spreading with age fairly stout stems having moderately-sized floral buds -medium growth rate (often in pairs at the terminus of the season's growth) Culture and smaller vegetative buds (as lateral buds on the -full sun to partial shade flowering stems, or as lateral and terminal buds on -best performance occurs in full sun in moist, well- portions of the shrub that are more shaded) drained, neutral to slightly acidic soils of average Trunk fertility, in areas with good air circulation; it is highly -multi-trunked, light brown, and slightly exfoliating adaptable to poor soils, soils of various pH, drought, in thin strips with maturity, becoming somewhat and pollution, but declines under the heat and high leggy with age, and with basal suckers having a rapid humidity of the Southern U.S. -
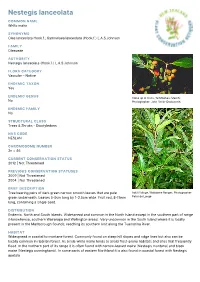
Nestegis Lanceolata
Nestegis lanceolata COMMON NAME White maire SYNONYMS Olea lanceolata Hook.f.; Gymnelaea lanceolata (Hook.f.) L.A.S.Johnson FAMILY Oleaceae AUTHORITY Nestegis lanceolata (Hook.f.) L.A.S.Johnson FLORA CATEGORY Vascular – Native ENDEMIC TAXON Yes ENDEMIC GENUS Close up of fruits, Te Moehau (March). No Photographer: John Smith-Dodsworth ENDEMIC FAMILY No STRUCTURAL CLASS Trees & Shrubs - Dicotyledons NVS CODE NESLAN CHROMOSOME NUMBER 2n = 46 CURRENT CONSERVATION STATUS 2012 | Not Threatened PREVIOUS CONSERVATION STATUSES 2009 | Not Threatened 2004 | Not Threatened BRIEF DESCRIPTION Tree bearing pairs of dark green narrow smooth leaves that are pale Adult foliage, Waitakere Ranges. Photographer: green underneath. Leaves 5-9cm long by 1-2.5cm wide. Fruit red, 8-11mm Peter de Lange long, containing a single seed. DISTRIBUTION Endemic. North and South Islands. Widespread and common in the North Island except in the southern part of range (Horowhenua, southern Wairarapa and Wellington areas). Very uncommon in the South Island where it is locally present in the Marlborough Sounds, reaching its southern limit along the Tuamarina River. HABITAT Widespread in coastal to montane forest. Commonly found on steep hill slopes and ridge lines but also can be locally common in riparian forest. As a rule white maire tends to avoid frost-prone habitats and sites that frequently flood. In the northern part of its range it is often found with narrow-leaved maire (Nestegis montana) and black maire (Nestegis cunninghamii). In some parts of eastern Northland it is also found in coastal forest with Nestegis apetala. FEATURES Stout gynodioecious spreading tree up to 20 m tall usually forming a domed canopy; trunk up to c. -

Crapemyrtle Bark Scale Acanthococcus (=Eriococcus) Lagerstroemiae
Crapemyrtle Bark Scale Acanthococcus (=Eriococcus) lagerstroemiae Jim Robbins, Univ. of Ark. CES, Bugwood.org, #5521505 The crapemyrtle bark scale (Acanthococcus (=Eriococcus) lagerstroemiae) is in the family Eriococcidae (or Acanthococcidae, as the taxonomy of this family is still being debated). This group is in the superfamily Coccoidea (scale insects) and the order Hemiptera (true bugs). The primary host in North America, crapemyrtle, Lagerstroemia spp., are deciduous flowering trees popular in ornamental landscapes. They are top-sellers in the nursery trade, with the annual wholesale value estimated to be $66 million in 2014. Based on urban tree inventories of several major cities in the southeastern U.S., crapemyrtle are among the most common landscape trees planted in this region. Resources - Cai, X., H. Dou, M. Gu, M. Merchant, and E. Vafaie. 2015. Update on crapymyrtle bark scale. Proceedings of the 2015 Annual Meeting of the International Plant Propagators’ Society. 1140: 415–418. - Wang, Z., Y. Chen, M. Gu, E. Vafaie, M. Merchant, and R. Diaz. 2016. Crapemyrtle bark scale: A new threat for crapemyrtles, a popular landscape plant in the U.S. Insects. 7: 33–34. 1 Crapemyrtle Bark Scale Close-up of a crapemyrtle bark scale • In the group of insects infestation, showing the fuzzy, felt-like known as felt scales covering of the adult female scales • Native to Asia • First report in the US – 2004 in Richardson, Texas (Dallas County) • Formerly known as Eriococcus lagerstroemiae Kuwana Gary Brooks, Bayer CropScience, Bugwood.org #5523058 The crapemyrtle bark scale (abbreviated as CMBS) belongs to a group of scale insects known as felt scales or bark scales. -

Developmental Biology of Leptoypha Mutica (Hemiptera: Tingidae) on Chinese Privet (Lamiales: Oleaceae) Author(S): J
Developmental Biology of Leptoypha mutica (Hemiptera: Tingidae) on Chinese Privet (Lamiales: Oleaceae) Author(s): J. Kalina, S.K. Braman, and J.L. Hanula Source: Journal of Entomological Science, 52(2):154-160. Published By: Georgia Entomological Society https://doi.org/10.18474/JES16-28.1 URL: http://www.bioone.org/doi/full/10.18474/JES16-28.1 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. Developmental Biology of Leptoypha mutica (Hemiptera: Tingidae) on Chinese Privet (Lamiales: Oleaceae)1 J. Kalina2, S.K. Braman3, and J.L. Hanula4 University of Georgia, Department of Entomology, 413 Biological Sciences Building, Athens, Georgia 30602 USA J. Entomol. Sci. 52(2): 154–160 (April 2017) Abstract The native lace bug, Leptoypha mutica Say (Hemiptera: Tingidae), has demonstrated potential as an insect biological control agent of invasive Chinese privet (Ligustrum sinense Lour). -

Studies on the Trichomes of Some Oleaceae, Structure and Ontogeny
STUDIES ON THE TRICHOMES OF SOME OLEACEAE, STRUCTURE AND ONTOGENY B~ J. A. INAMDAR (Department of Botany, Sardar Patel Univeraity, Vallabh Vidyanagar, Gujarat) Received August 22, 1967 (Communicated by Prof. V. Puff, r.A.sc.) ABSTRACT Some twelve types of tricho~aes are described for four species of Jasminum and Nyctanthes arbor-tristis L. They fall under two main categories, the eglandular and the glandular trichomes. The systematic position of Nyctanthes seems to be quite natural in the family Oleaceae. INTRODUCTION The family Oleaceae has received considerable attention of the botanists particularly with reference to the systcmatic position of Nyctanthes and the monotypic genus Dimetra. Airy SEaw (1952) transferred these gcnera to the Verbenaceae on morphological grounds. Stant (1952) has supported Airy Shaw on anatomical grounds. Johnson (1957) in a review of the family Oleaceae opined that Nyctanthes should be excluded from Oleaceae based on the cytological findings of Taylor (1945). On the other hand the embryo- logical data (Patel, 1963; Kapil, 1967) suggests that Nyctanthes should be retained in the Oleaceae. Several workers (De Bary, 1884; Cowan, 1950, Goodspeed, 1954; Carlquist, 1958, 1959a, 1959b, 1959c, 1961; Mathur, 1961 ; Ramayya, 1962 a, 1962b, 1963) have described the structure and development of trichomes and glands in several angisoperm families. The present investi- gation was carried out to find if the trichomes ~nd glands could be of any help in settling the doubtful systematic position of Oleaceous genera. The present paper deals with the study of eglandular and glandular (glands) trichomes on the vegetative organs of four species of Jasminum, viz., J. -

The Vegetation of the Western Blue Mountains Including the Capertee, Coxs, Jenolan & Gurnang Areas
Department of Environment and Conservation (NSW) The Vegetation of the Western Blue Mountains including the Capertee, Coxs, Jenolan & Gurnang Areas Volume 1: Technical Report Hawkesbury-Nepean CMA CATCHMENT MANAGEMENT AUTHORITY The Vegetation of the Western Blue Mountains (including the Capertee, Cox’s, Jenolan and Gurnang Areas) Volume 1: Technical Report (Final V1.1) Project funded by the Hawkesbury – Nepean Catchment Management Authority Information and Assessment Section Metropolitan Branch Environmental Protection and Regulation Division Department of Environment and Conservation July 2006 ACKNOWLEDGMENTS This project has been completed by the Special thanks to: Information and Assessment Section, Metropolitan Branch. The numerous land owners including State Forests of NSW who allowed access to their Section Head, Information and Assessment properties. Julie Ravallion The Department of Natural Resources, Forests NSW and Hawkesbury – Nepean CMA for Coordinator, Bioregional Data Group comments on early drafts. Daniel Connolly This report should be referenced as follows: Vegetation Project Officer DEC (2006) The Vegetation of the Western Blue Mountains. Unpublished report funded by Greg Steenbeeke the Hawkesbury – Nepean Catchment Management Authority. Department of GIS, Data Management and Database Environment and Conservation, Hurstville. Coordination Peter Ewin Photos Kylie Madden Vegetation community profile photographs by Greg Steenbeeke Greg Steenbeeke unless otherwise noted. Feature cover photo by Greg Steenbeeke. All Logistics -

Intraspecific, Interspecific, and Interseries Cross-Compatibility in Lilac
J. AMER.SOC.HORT.SCI. 142(4):279–288. 2017. doi: 10.21273/JASHS04155-17 Intraspecific, Interspecific, and Interseries Cross-compatibility in Lilac Jason D. Lattier 1 and Ryan N. Contreras 2 Department of Horticulture, 4017 Agriculture and Life Sciences Building, Oregon State University, Corvallis, OR 97331-7304 ADDITIONAL INDEX WORDS. Syringa, Pubescentes, Villosae, in vitro germination, controlled crosses, wide hybridization ABSTRACT. Lilacs (Syringa sp.) are a group of ornamental trees and shrubs in the Oleaceae composed of 22–30 species from two centers of diversity: the highlands of East Asia and the Balkan-Carpathian region of Europe. There are six series within the genus Syringa: Pubescentes, Villosae, Ligustrae, Ligustrina, Pinnatifoliae, and Syringa. Intraspecific and interspecific hybridization are proven methods for cultivar development. However, reports of interseries hybridization are rare and limited to crosses among taxa in series Syringa and Pinnatifoliae. Although hundreds of lilac cultivars have been introduced, fertility and cross-compatibility have yet to be formally investigated. Over 3 years, a cross-compatibility study was performed using cultivars and species of shrub-form lilacs in series Syringa, Pubescentes, and Villosae. A total of 114 combinations were performed at an average of 243 ± 27 flowers pollinated per combination. For each combination, we recorded the number of inflorescences and flowers pollinated as well as number of capsules, seed, seedlings germinated, and albino seedlings. Fruit and seed were produced from interseries crosses, but no seedlings were recovered. A total of 2177 viable seedlings were recovered from interspecific and intraspecific combinations in series Syringa, Pubescentes, and Villosae. Albino progeny were produced only from crosses with Syringa pubescens ssp. -

Ist Β-Glucosidase Ein Schlüsselenzym Für Die
Ist β-Glucosidase ein Schlüsselenzym für die Anpassung an Iridoidglycoside? Ein Vergleich zwischen herbivoren Generalisten (Arctiidae, Lepidoptera) und einem Iridoidglycosidspezialist (Nymphalidae, Lepidoptera) Dissertation zur Erlangung der Doktorgrades der Naturwissenschaften (Dr. rer. nat.) im Department Biologie der Fakultät für Mathematik, Informatik und Naturwissenschaften an der Universität Hamburg vorgelegt von Helga Carola Pankoke aus Freiburg im Breisgau Hamburg 2010 Erstgutachterin: Professor Dr. Susanne Dobler Zweitgutachter: Professor Dr . Reinhard Lieberei Tag der Disputation: 9. April 2010 Bestätigung zur sprachlichen Korrektheit der in englischer Sprache abgefassten Dissertation Die sprachliche Korrektheit der in englischer Sprache abgefassten Dissertation von Frau Helga Carola Pankoke mit wurde von der Unterzeichnenden geprüft und bestätigt. (engl . „The grammatical and linguistical correctness of the dissertation, which has been written in English by Ms. Helga Carola Pankoke, has been proved and confirmed.“) (M. Deane Bowers) Museum and Department of Ecology and Evolutionary Biology, UCB 334 University of Colorado, Boulder Colorado 80309, USA Inhaltsverzeichnis Zusammenfassung i 1 Allgemeine Einleitung 01 2 Influence of iridoid glycoside containing host plants on midgut β-glucosidase activity in the yellow woolly bear, Spilosoma virginica (Arctiidae) 06 2.1 Summary 06 2.2 Introduction 07 2.3 Materials and Methods 10 2.4 Results 14 2.5 Discussion 19 3 The interplay between toxin-releasing β-glucosidase and plant iridoid