Visualization and Quantification of Mimetic Musculature Via Dicect
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Vascular Supply of the Human Spiral Ganglion: Novel Three
www.nature.com/scientificreports Corrected: Publisher Correction OPEN Vascular Supply of the Human Spiral Ganglion: Novel Three- Dimensional Analysis Using Synchrotron Phase-Contrast Imaging and Histology Xueshuang Mei1,2*, Rudolf Glueckert3, Annelies Schrott-Fischer3, Hao Li1, Hanif M. Ladak4,6, Sumit K. Agrawal5,6 & Helge Rask-Andersen1,6* Human spiral ganglion (HSG) cell bodies located in the bony cochlea depend on a rich vascular supply to maintain excitability. These neurons are targeted by cochlear implantation (CI) to treat deafness, and their viability is critical to ensure successful clinical outcomes. The blood supply of the HSG is difcult to study due to its helical structure and encasement in hard bone. The objective of this study was to present the frst three-dimensional (3D) reconstruction and analysis of the HSG blood supply using synchrotron radiation phase-contrast imaging (SR-PCI) in combination with histological analyses of archival human cochlear sections. Twenty-six human temporal bones underwent SR-PCI. Data were processed using volume-rendering software, and a representative three-dimensional (3D) model was created to allow visualization of the vascular anatomy. Histologic analysis was used to verify the segmentations. Results revealed that the HSG is supplied by radial vascular twigs which are separate from the rest of the inner ear and encased in bone. Unlike with most organs, the arteries and veins in the human cochlea do not follow the same conduits. There is a dual venous outfow and a modiolar arterial supply. This organization may explain why the HSG may endure even in cases of advanced cochlear pathology. Human inner ear function relies on microcirculation derived from vessels in the internal auditory canal (IAC). -

Cochlear Partition Anatomy and Motion in Humans Differ from The
Cochlear partition anatomy and motion in humans differ from the classic view of mammals Stefan Raufera,b,1, John J. Guinan Jra,b,c, and Hideko Heidi Nakajimaa,b,c aEaton-Peabody Laboratories, Massachusetts Eye and Ear, Boston, MA 02114; bSpeech and Hearing Bioscience and Technology Program, Harvard University, Cambridge, MA 02138; and cDepartment of Otolaryngology, Harvard Medical School, Boston, MA 02115 Edited by Christopher A. Shera, University of Southern California, Los Angeles, CA, and accepted by Editorial Board Member Thomas D. Albright June 6, 2019 (received for review January 16, 2019) Mammals detect sound through mechanosensitive cells of the assume there is no OSL motion (10–13). Additionally, the attach- cochlear organ of Corti that rest on the basilar membrane (BM). ment of the OSL to the BM and the attachment of the TM to Motions of the BM and organ of Corti have been studied at the spiral limbus (which sits above the OSL) are also consid- the cochlear base in various laboratory animals, and the assump- ered stationary. In contrast to this view, there have been reports tion has been that the cochleas of all mammals work similarly. of sound-induced OSL movement, but these reports have been In the classic view, the BM attaches to a stationary osseous spi- largely ignored in overviews of cochlear mechanics and the for- ral lamina (OSL), the tectorial membrane (TM) attaches to the mation of cochlear models (10–13). Von B´ek´esy (14), using static limbus above the stationary OSL, and the BM is the major mov- pressure, found that the CP “bent like an elastic rod that was free ing element, with a peak displacement near its center. -

ANATOMY of EAR Basic Ear Anatomy
ANATOMY OF EAR Basic Ear Anatomy • Expected outcomes • To understand the hearing mechanism • To be able to identify the structures of the ear Development of Ear 1. Pinna develops from 1st & 2nd Branchial arch (Hillocks of His). Starts at 6 Weeks & is complete by 20 weeks. 2. E.A.M. develops from dorsal end of 1st branchial arch starting at 6-8 weeks and is complete by 28 weeks. 3. Middle Ear development —Malleus & Incus develop between 6-8 weeks from 1st & 2nd branchial arch. Branchial arches & Development of Ear Dev. contd---- • T.M at 28 weeks from all 3 germinal layers . • Foot plate of stapes develops from otic capsule b/w 6- 8 weeks. • Inner ear develops from otic capsule starting at 5 weeks & is complete by 25 weeks. • Development of external/middle/inner ear is independent of each other. Development of ear External Ear • It consists of - Pinna and External auditory meatus. Pinna • It is made up of fibro elastic cartilage covered by skin and connected to the surrounding parts by ligaments and muscles. • Various landmarks on the pinna are helix, antihelix, lobule, tragus, concha, scaphoid fossa and triangular fossa • Pinna has two surfaces i.e. medial or cranial surface and a lateral surface . • Cymba concha lies between crus helix and crus antihelix. It is an important landmark for mastoid antrum. Anatomy of external ear • Landmarks of pinna Anatomy of external ear • Bat-Ear is the most common congenital anomaly of pinna in which antihelix has not developed and excessive conchal cartilage is present. • Corrections of Pinna defects are done at 6 years of age. -

Organum Vestibulocochleare INTERNAL EAR MIDDLE EAR EXTERNAL EAR PETROSAL BONE- Eq EXTERNAL EAR AURICLE
EAR organum vestibulocochleare INTERNAL EAR MIDDLE EAR EXTERNAL EAR PETROSAL BONE- Eq EXTERNAL EAR AURICLE The external ear plays the role of an acoustic antenna: auricle the auricle (together with the head) collects and focuses sound waves, the ear canal act as a resonator. tympanic membrane anular cartilage meatus acusticus externus EXTERNAL EAR EXTERNAL EAR AURICLE scutiform cartilage Auricular muscles: -Dorsal -Ventral -Rostral -Caudal EXTERNAL EAR MEATUS ACUSTICUS EXTERNUS auricular cartilage vertical canal auditory ossicles horizontal cochlea canal auditory tube tympanic tympanic eardrum bulla cavity tympanic membrane MIDDLE EAR Auditory ossicles STAPES INCUS Tympanic cavity: (anvil) (stirrup) - epitympanium - mesotympanium - hypotympanium MALLEUS (hammer) auditory vestibular window- ossicles or oval window through which mechanical stimuli (transmitted by the auditory ossicles) enter the epitympanic internal ear for translation recess into nerve impulses auditory tube (Eustachian tube) cochlear window- or round window tympanic cavity bulla tympanica through which the vibration of the perilympha is absorbed MIDDLE EAR MIDDLE EAR GUTTURAL POUCH- Eq MIDDLE EAR AUDITORY OSSICLES head INCUS processus rostralis (stirrup) STAPES processus muscularis (anvil) manubrium short crus body MALLEUS (hammer) Two muscles of the ossicles: long crus m. tensor tympani- n. tensoris tympani ex. n. base mandibularis (footplate) m. stapedius- n. stapedius ex. n. facialis crus The muscles fix the bones and protect the cochlea crus against the harmful effects -

Temporal Bone, Normal Anatomy, Computed Tomography/Histology
Temporal Bone Normal Anatomy Computed Tomography /Histology Correlation Otopathology Laboratory Department of Radiology Massachusetts Eye and Ear Eric F. Curtin Barbara J. Burgess Dianne D. Jones Jennifer T. O’Malley Hugh D. Curtin MD Otopathologylaboratory.org Facial nerve Malleus Incus Cochlea Lateral canal Oval Window Axial CT Red Arrow -Facial nerve VII Attic IAC Vest LSCC Red Arrow -Facial nerve VII M I M-Malleus I-Incus Vest Cochlea S-Stapes VII S Interscalar septum M M-Modiolus Interscalar septum M M-Modiolus Anterior epitympanic recess Cog Cochleariform process Cochlea Facial recess S-Stapes Pyramidal process S Sinus Tympani ME ME-middle ear RW-round window Cochlea RW Stapedius M Carotid TMJ plate Jugular plate Coronal CT FN 2 FN 1 Cochlea Carotid Malleus Tegmen Incus FN 2 Oval window Scutum Lateral Semicircular Canal Pyramidal process Round Window Jugular Facial Nerve Key image lateral canal facial nerve oval window .. Poschl CT Carotid Tensor tympani Eustachian tube VII1 IAC Basilar turn cochlea Apical turn cochlea Turn 2 cochlea VII1 VII2 Tensor tympani VII2 Jugular vein Tensor tympani Jugular vein Cochleariform process Superior SCC M M-malleus VII2 Oval W Round W Lateral canal Common crus TMJ Lateral SCC VII2 I S I –Incus S- Stapes Tegmen Vestibular aqueduct VII2 VII3 EAC Stenvers CT SSCC VII1 Carotid VII1 is separated from nerve to lateral canal by Bill’s bar Canal for nerve to lateral SCC VII VII1 Basilar turn cochlea LSCC SSCC VII3 Round window Styloid process Vestibule VII3 PSCC Common Crus IAC Axial CT Histology correlation Utricle Lateral Semicircular Canal Vestibule Vestibule Saccule Utricle Vestibule Saccule Utricle Vestibule Saccule Utricle Anterior Crus of the Stapes Carotid Tympanic Carotid plate membrane Middle Ear Jugular Jugular plate Coronal CT Histology correlation Saccule Otopathology Laboratory Department of Radiology Massachusetts Eye and Ear Otopathologylaboratory.org Eric F. -

Anatomic Moment
Anatomic Moment Hearing, I: The Cochlea David L. Daniels, Joel D. Swartz, H. Ric Harnsberger, John L. Ulmer, Katherine A. Shaffer, and Leighton Mark The purpose of the ear is to transform me- cochlear recess, which lies on the medial wall of chanical energy (sound) into electric energy. the vestibule (Fig 3). As these sound waves The external ear collects and directs the sound. enter the perilymph of the scala vestibuli, they The middle ear converts the sound to fluid mo- are transmitted through the vestibular mem- tion. The inner ear, specifically the cochlea, brane into the endolymph of the cochlear duct, transforms fluid motion into electric energy. causing displacement of the basilar membrane, The cochlea is a coiled structure consisting of which stimulates the hair cell receptors of the two and three quarter turns (Figs 1 and 2). If it organ of Corti (Figs 4–7) (4, 5). It is the move- were elongated, the cochlea would be approxi- ment of hair cells that generates the electric mately 30 mm in length. The fluid-filled spaces potentials that are converted into action poten- of the cochlea are comprised of three parallel tials in the auditory nerve fibers. The basilar canals: an outer scala vestibuli (ascending spi- membrane varies in width and tension from ral), an inner scala tympani (descending spi- base to apex. As a result, different portions of ral), and the central cochlear duct (scala media) the membrane respond to different auditory fre- (1–7). The scala vestibuli and scala tympani quencies (2, 5). These perilymphatic waves are contain perilymph, a substance similar in com- transmitted via the apex of the cochlea (helico- position to cerebrospinal fluid. -
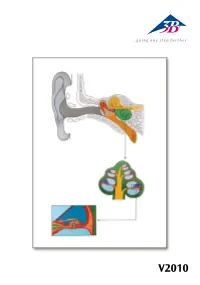
B005DTJC8Y.Usermanual.Pdf
V2010 Auris Latin Auris externa 1 Auricula 2 Meatus acusticus externus 3 Membrana tympanica Auris media 4 Ossicula auditus: 4 a Malleus 4 b Incus 4 c Stapes 5 Cavitas tympani 6 Tuba auditiva 7 Fenestra vestibuli 8 Fenestra cochleae Auris interna 9 Labyrinthus osseus: 9 a Canalis semicircularis posterior 9 b Canalis semicircularis lateralis 9 c Canalis semicircularis anterior 9 d Vestibulum 9 e Cochlea 10 N. vestibularis 11 N. cochlearis ® 12 N. vestibulocochlearis [VIII] 13 Lig. spirale 14 Scala vestibuli 15 Ductus cochlearis 16 Scala tympani 17 N. cochlearis 18 Ganglion cochleare 19 Organum spirale 20 Modiolus cochleae 21 Paries vestibularis 22 Sulcus spiralis internus 23 Membrana tectoria 24 Cuniculus medius 25 Cuniculus externus 26 Cellulae terminales externae 27 Cellulae sustentaculares externae 28 Sulcus spiralis externus 29 Lamina basilaris 30 Cellulae phalangea externae 31 Cellulae capillares externae 32 Cellula stela externa 33 Cuniculus internus 34 Cellula capillaris interna 35 Cellula stela interna 36 Lamina spiralis ossea 37 Limbus spiralis English The Ear A Sectional view of the human ear B Sectional view of cochlea C Sectional view of cochlear duct with spiral organ External ear 1 Auricle 2 External acoustic meatus 3 Tympanic membrane Middle ear 4 Auditory ossicles: 4 a Malleus 4 b Incus 4 c Stapes 5 Tympanic cavity 6 Pharyngotympanice tube 7 Oval window 8 Round window Internal ear 9 Bony labyrinth 9 a Posterior semicircular canal 9 b Lateral semicircular canal 9 c Anterior semicircular canal ® 9 d Vestibule 9 e Cochlea 10 -

Role of Mitochondrial Uncoupling Protein 4 in Rat Inner Ear
Molecular and Cellular Neuroscience 47 (2011) 244–253 Contents lists available at ScienceDirect Molecular and Cellular Neuroscience journal homepage: www.elsevier.com/locate/ymcne Role of mitochondrial uncoupling protein 4 in rat inner ear Alina Smorodchenko a,c, Anne Rupprecht a,c, Julia Fuchs b, Johann Gross b, Elena E. Pohl a,c,⁎ a Department of Physiology and Biophysics, University of Veterinary Medicine, Vienna, Austria b Department of Othorhinolaryngology, Charité—Universitaetsmedizin Berlin, Germany c Institute of Cell Biology and Neurobiology, Charité—Universitaetsmedizin Berlin, Germany article info abstract Article history: The uncoupling protein 4 (UCP4) belongs to the mitochondrial anion transporter family. Protein tissue Received 3 September 2010 distribution and functions are still a matter of debate. Using an antibody we have previously shown that UCP4 Revised 3 March 2011 appears in neurons and to a lesser extent in astrocytes of murine neuronal tissue as early as days 12–14 of Accepted 3 March 2011 embryonic development (Smorodchenko et al., 2009). Here we demonstrated for the first time that Available online 11 March 2011 neurosensory cells such as hair cells of the inner ear and mechanosensitive Merkel cells in skin also express a significant amount of UCP4. We tested the hypothesis about whether UCP4 contributes to the regulation of Keywords: Mitochondrial membrane protein oxidative stress using the model of oxygen deprivation. For this we compared the protein expression level in Voltage dependent anion channel freshly isolated explants of organ of Corti, modiolus and stria vascularis from neonatal rats with explants Spiral ganglion neurons cultured under hypoxia. Western blot analysis revealed that the UCP4 level was not increased under hypoxic Cochlea development conditions, when compared to the mitochondrial outer membrane protein VDAC or to the anti-oxidative Hypoxia enzyme SOD2. -

Evaluation of Human Ear Anatomy and Functionality by Axiomatic Design
biomimetics Article Evaluation of Human Ear Anatomy and Functionality by Axiomatic Design Pratap Sriram Sundar 1, Chandan Chowdhury 2 and Sagar Kamarthi 3,* 1 Indian School of Business, Mohali 160062, India; [email protected] 2 Indian School of Business, Gachibowli, Hyderabad 500111, India; [email protected] 3 Department of Mechanical and Industrial Engineering, Northeastern University, Boston, MA 02115, USA * Correspondence: [email protected]; Tel.: +1-617-373-3070 Abstract: The design of the human ear is one of nature’s engineering marvels. This paper examines the merit of ear design using axiomatic design principles. The ear is the organ of both hearing and balance. A sensitive ear can hear frequencies ranging from 20 Hz to 20,000 Hz. The vestibular apparatus of the inner ear is responsible for the static and dynamic equilibrium of the human body. The ear is divided into the outer ear, middle ear, and inner ear, which play their respective functional roles in transforming sound energy into nerve impulses interpreted in the brain. The human ear has many modules, such as the pinna, auditory canal, eardrum, ossicles, eustachian tube, cochlea, semicircular canals, cochlear nerve, and vestibular nerve. Each of these modules has several subparts. This paper tabulates and maps the functional requirements (FRs) of these modules onto design parameters (DPs) that nature has already chosen. The “independence axiom” of the axiomatic design methodology is applied to analyze couplings and to evaluate if human ear design is a good design (i.e., uncoupled design) or a bad design (i.e., coupled design). The analysis revealed that the human ear is a perfect design because it is an uncoupled structure. -

Tympanic Membrane (Membrana Tympanica, Myrinx)
Auditory and vestibular system Auris, is = Us, oton Auditory and vestibular system • external ear (auris externa) • middle ear (auris media) • internal ear (auris interna) = organum vestibulo- cochleare External ear (Auris externa) • auricle (auricula, pinna) – elastic cartilage • external acoustic meatus (meatus acusticus externus) • tympanic membrane (membrana tympanica, myrinx) • helix Auricle – crus, spina, cauda – (tuberculum auriculare Darwini, apex auriculae) • antihelix – crura, fossa triangularis • scapha • concha auriculae – cymba, cavitas • tragus • antitragus • incisura intertragica • lobulus auriculae posterior surface = negative image of the anterior one ligaments: lig. auriculare ant., sup., post. muscles – innervation: n. facialis • extrinsic muscles = facial muscles – mm. auriculares (ant., sup., post.) – m. temporoparietalis • intrinsic muscles: rudimentary – m. tragicus + antitragicus – m. helicis major+minor – m. obliquus + transversus auriculae, m. pyramidalis auriculae cartilage: cartilago auriculae - elastic skin: dorsally more loosen, ventrally firmly fixed to perichondrium - othematoma Auricle – supply • arteries: a. temporalis superficialis → rr. auriculares ant. a. carotis externa → a. auricularis post. • veins: v. jugularis ext. • lymph: nn.ll. parotidei, mastoidei • nerves: sensory – nn. auriculares ant. from n. auriculotemporalis (ventrocranial 2/3) – r. auricularis n. X. (concha) – n. occipitalis minor (dosrocranial) – n. auricularis magnus (cudal) motor: n. VII. External acoustic meatus (meatus acusticus -

Inner Ear Malformations: a Practical Diagnostic Approachଝ
Document downloaded from http://www.elsevier.es, day 09/03/2018. This copy is for personal use. Any transmission of this document by any media or format is strictly prohibited. Radiología. 2017;59(4):297---305 www.elsevier.es/rx RADIOLOGY THROUGH IMAGES Inner ear malformations: A practical diagnostic approachଝ a,∗ b a a a M. Mazón , E. Pont , A. Montoya-Filardi , J. Carreres-Polo , F. Más-Estellés a Área Clínica de Imagen Médica, Hospital Universitario y Politécnico La Fe, Valencia, Spain b Servicio de Otorrinolaringología, Hospital General de Onteniente, Valencia, Spain Received 20 July 2016; accepted 27 September 2016 KEYWORDS Abstract Pediatric sensorineural hearing loss is a major cause of disability; although inner ear Diagnostic imaging; malformations account for only 20---40% of all cases, recognition and characterization will be Ear inner; vital for the proper management of these patients. In this article relevant anatomy and devel- Abnormalities; opment of inner ear are surveyed. The role of neuroimaging in pediatric sensorineural hearing Cochlear displasia; loss and cochlear preimplantation study are assessed. The need for a universal system of classi- Cochlear aplasia; fication of inner ear malformations with therapeutic and prognostic implications is highlighted. Incomplete partition And finally, the radiological findings of each type of malformation are concisely described and depicted. Computed tomography and magnetic resonance imaging play a crucial role in the char- acterization of inner ear malformations and allow the assessment of the anatomical structures that enable the selection of appropriate treatment and surgical approach. © 2016 SERAM. Published by Elsevier Espana,˜ S.L.U. All rights reserved. PALABRAS CLAVE Malformaciones del oído interno: una aproximación diagnóstica práctica Imagen diagnóstica; Oído interno; Resumen La hipoacusia neurosensorial pediátrica es una causa mayor de discapacidad. -

Temporal Bone Anatomy
Temporal Bone Anatomy C. Kirsch M.D. [email protected] Assistant Professor of Neuroradiology and Head and Neck Radiology David Geffen School of Medicine at UCLA Goals of this lecture • To review the key anatomy in both the axial and coronal plane • Test your knowledge of that anatomy • The importance and revelance of the structure identified! Axial CT Scan –Right side Key structures • Internal carotid artery Axial CT Scan –Right side • Internal carotid artery • Internal jugular vein Axial CT Scan –Right side •Internal carotid artery • Internal jugular vein •Sigmoid sinus Axial CT Scan –Right side QuickTime™ and a decompressor are needed to see this picture. BB = Bill’s bar TC- Falciform or transverse crest Think - Seven up Coke down QuickTime™ and a decompressor are needed to see this picture. Internal auditory canal contains the intracanicular segment of the facial nerve (VII) and the vestibulocochlear nerve (VIII) Axial CT Scan –Right side QuickTime™ and a decompressor are needed to see this picture. Going to follow the course of the facial nerve! First portion ‐ fundus of the IAC Facial Nerve‐ Key to T‐bone! Slides courtesy of Amerisys Facial Nerve‐ Key to T‐bone! Slides courtesy of Amerisys Facial Nerve‐ Key to T‐bone! Superior salivatory nucleus parasympathetics Solitary tract Motor nucleus CN VII nucleus Lateral SCC Stapedius n. GSPN - lacrimal gland Stylomastoid foramen Chorda tympani nerve - parasymp -SMG, SLG Extracranial Taste ant 2/3 tongue motor CN 7 Slides courtesy of Amerisys Facial Nerve‐ Key to T‐bone! Solitary tract Motor nucleus CN VII nucleus Superior salivatory nucleus parasympathetics Lateral SCC GSPN - lacrimal gland Stapedius n.