Downloads/Netzwerk Und Leopold Voss, Hamburg, 1904
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Annual Report 2013.Pdf
ATOMIC HERITAGE FOUNDATION Preserving & Interpreting Manhattan Project History & Legacy preserving history ANNUAL REPORT 2013 WHY WE SHOULD PRESERVE THE MANHATTAN PROJECT “The factories and bombs that Manhattan Project scientists, engineers, and workers built were physical objects that depended for their operation on physics, chemistry, metallurgy, and other nat- ural sciences, but their social reality - their meaning, if you will - was human, social, political....We preserve what we value of the physical past because it specifically embodies our social past....When we lose parts of our physical past, we lose parts of our common social past as well.” “The new knowledge of nuclear energy has undoubtedly limited national sovereignty and scaled down the destructiveness of war. If that’s not a good enough reason to work for and contribute to the Manhattan Project’s historic preservation, what would be? It’s certainly good enough for me.” ~Richard Rhodes, “Why We Should Preserve the Manhattan Project,” Bulletin of the Atomic Scientists, May/June 2006 Photographs clockwise from top: J. Robert Oppenheimer, General Leslie R. Groves pinning an award on Enrico Fermi, Leona Woods Marshall, the Alpha Racetrack at the Y-12 Plant, and the Bethe House on Bathtub Row. Front cover: A Bruggeman Ranch property. Back cover: Bronze statues by Susanne Vertel of J. Robert Oppenheimer and General Leslie Groves at Los Alamos. Table of Contents BOARD MEMBERS & ADVISORY COMMITTEE........3 Cindy Kelly, Dorothy and Clay Per- Letter from the President..........................................4 -

2006Spring.Pdf
− X8 PROTEUM THE ULTIMATE STRUCTURAL BIOLOGY SYSTEM When you need the best system for Structural Biology, the Bruker X8 PROTEUM offers high-throughput screening AND superb high resolution data in one uncompromising package. With our MICROSTAR family of generators, you can rely on the extremely intense micro-focus X-ray beam coupled with the ultra-bright HELIOS optics to handle everything from small crystals to large unit cells With over 700135 detector CCD detectors for speed, installed, sensitivity, we know size and how dynamic to optimize range the to give PLATINUM you the best data possible in the home lab Our KAPPA goniometer’s high precision mechanics allow you to orient the sample along any axis in reciprocal space, while having easy access to mount, cool or anneal your crystals Get the best data, get the fastest system, get the power to solve your structures – X8 PROTEUM. BRUKER ADVANCED X-RAY SOLUTIONS North America: BRUKER AXS INC Tel. (+1) (608) 276-3000 Fax (+1) (608) 276-3006 www.bruker-axs.com [email protected] Germany: BRUKER AXS GMBH Tel. (+49) (721) 595- 2888 Fax (+49) (721) 595-4587 www.bruker-axs.de [email protected] Netherlands: BRUKER AXS BV Tel. (+31) (15) 215-2400 Fax (+31) (15) 215-2500 www.bruker-axs.nl [email protected] American Crystallographic Association * REFLECTIONS *see page 9 for notes on our new name and for new logo possibilities Cover: Images from Warren Award Recipient Charles Majkrazk and his colleagues; see page 25. ACA HOME PAGE: hwi.buffalo.edu/ACA/ Table of Contents 3 President’s -

Robert Wilhelm Bunsen Und Sein Heidelberger Laboratorium Heidelberg, 12
Historische Stätten der Chemie Robert Wilhelm Bunsen und sein Heidelberger Laboratorium Heidelberg, 12. Oktober 2011 Gesellschaft Deutscher Chemiker 1 Mit dem Programm „Historische Stätten der Chemie“ würdigt Robert Wilhelm Bunsen – die Gesellschaft Deutscher Chemiker (GDCh) Leistungen von geschichtlichem Rang in der Chemie. Als Orte der Erinnerung eine biographische Skizze werden Wirkungsstätten beteiligter Wissenschaftlerinnen und Wissenschaftler in einem feierlichen Akt ausgezeichnet. Eine Broschüre bringt einer breiten Öffentlichkeit deren wissenschaft- Bunsen war einer der Wegbereiter der Physikalischen Chemie liches Werk näher und stellt die Tragweite ihrer Arbeiten im und ein bedeutender Vertreter der anorganisch-analytischen aktuellen Kontext dar. Ziel dieses Programms ist es, die Erinne- Richtung. Seine wissenschaftliche Bedeutung liegt in der Ent- rung an das kulturelle Erbe der Chemie wach zu halten und die wicklung und Perfektionierung von Methoden und Instrumen- Chemie mit ihren historischen Wurzeln stärker in das Blickfeld ten. Diese Arbeitsschwerpunkte hat Bunsen von Beginn seiner der Öffentlichkeit zu rücken. Karriere an verfolgt und systematisch ausgebaut. Am 12. Oktober 2011 gedenken die GDCh, die Deutsche 1811 als jüngster von vier Söhnen einer bürgerlichen protestan- Bunsen-Gesellschaft für Physikalische Chemie (DBG), die Che- tischen Familie in Göttingen geboren, begann Bunsen dort 1828 mische Gesellschaft zu Heidelberg (ChGzH) und die Ruprecht- das Studium der Naturwissenschaften. Seine wichtigsten Lehrer Karls-Universität -

ACA Structure Matters, Winter 2017
ACA Isabella Karle (1921-2017) Winter 2017 Structure Matters Remembering Isabella Karle “I need to tackle this scientific problem, what techniques are Isabella Karle (1921 - 2017), retired from the Naval Research available to help me do it?” and when she found out what they Laboratory (Washington, DC) after more than six decades there, were she would solve the problem. In this case she worked with passed away on October 3, 2017, at the age of 95, from a brain silica tubes that she had made, filled with crude plutonium oxide tumor. Early on Isabella was told by a teacher that chemistry and chemical reactants, and inserted them in a hole in a large was not a “proper field for girls” but she went on to become a block of copper that was heated to high temperatures of 800 to member of the National Academy of Sciences. She received the 900 degrees Centigrade. After many experiments under difficult 1988 Gregori Aminoff Prize from the Royal Swedish Academy conditions, she ended up, triumphantly, with bright green crystals of plutonium chloride (PuCl ) that she passed on to the physics of Sciences, the 1993 Bower Award and Prize for Achievement 3 in Science and, in 1995, received the National Medal of Science. branch of the Manhattan project. What follows are remembrances from several of her colleagues. Isabella approached direct methods in the same way, to the delight of her husband Jerry, who won the Nobel Prize for his work on them. She worked hard to find how to run direct methods correctly and then was able to help others. -

70Th Anniversary of the Manhattan Project Atomic Heritage Foundation
Atomic Heritage Foundation presents 70th Anniversary of the Manhattan Project June 2 and 3, 2015 Carnegie Institution for Science 1530 P Street, NW Washington, DC 20005 Visit our merchandise tables to purchase books, posters, and hats! Manhattan Project 70th Anniversary Manhattan Project veterans Lawrence S. O’Rourke (left) and William E. Tewes (right) with his future wife, Olive. The Atomic Heritage Foundation is proud to host events commemorating the 70th Anniversary of the Manhattan Project. It took more than half a million people to build the world’s first atomic bombs; we are honored to welcome more than a dozen men and women who participated in that astonishing effort. The 70th Anniversary Reunion on June 2 will be an opportunity for vet- erans and family members to share their memories and catch up with old friends. Veterans from Los Alamos, Oak Ridge, Hanford, Chicago and other locations will discuss how each site contributed to the Manhattan Project in its own unique way. The 70th Anniversary commemoration will continue on June 3 with a day- long symposium, which will feature a discussion of the new Manhattan Project National Historical Park. We have assembled a first-class roster of Manhattan Project veterans and experts who will discuss topics ranging from innovation to women in science to atomic spies and more. We hope you enjoy the events! Cynthia C. Kelly President, Atomic Heritage Foundation Atomic Heritage Foundation The Atomic Heritage Foundation (AHF), founded by Cynthia C. Kelly in 2002, is a nonprofit organization in Washington, DC, dedicated to the preservation and interpretation of the Manhattan Project and its legacy. -

SCIENCE HISTORY INSTITUTE ISABELLA KARLE and JEROME
SCIENCE HISTORY INSTITUTE ISABELLA KARLE and JEROME KARLE Transcript of an Interview Conducted by James J. Bohning and David K. Van Keuren at Naval Research Laboratory Washington, District of Columbia on 26 February, 15 June and 9 September 1987 (With Subsequent Corrections and Additions) Upon Isabella Karle’s death in 2017, this oral history was designated Free Access. Please note: This oral history is protected by U.S. copyright law and shall not be reproduced or disseminated in any way without the express permission of the Science History Institute. Users citing this interview for purposes of publication are obliged under the terms of the Center for Oral History, Science History Institute, to credit the Science History Institute using the format below: Isabella Karle and Jerome Karle, interview by James J. Bohning and David K. Van Keuren at Naval Research Laboratory, Washington, District of Columbia, 26 February, 15 June and 9 September 1987 (Philadelphia: Science History Institute, Oral History Transcript # 0066). Formed by the merger of the Chemical Heritage Foundation and the Life Sciences Foundation, the Science History Institute collects and shares the stories of innovators and of discoveries that shape our lives. We preserve and interpret the history of chemistry, chemical engineering, and the life sciences. Headquartered in Philadelphia, with offices in California and Europe, the Institute houses an archive and a library for historians and researchers, a fellowship program for visiting scholars from around the globe, a community of researchers who examine historical and contemporary issues, and an acclaimed museum that is free and open to the public. For more information visit sciencehistory.org. -

Calendario De Mujeres Científicas Y Maestras
Mil Jardines Ciencia y Tecnología Calendario de Mujeres Científicas y Maestras Hipatia [Jules Maurice Gaspard (1862–1919)] Por Antonio Clemente Colino Pérez [Contacto: [email protected]] CIENCIA Y TECNOLOGÍA Mil Jardines . - Calendario de Mujeres Científicas y Maestras - . 1 – ENERO Marie-Louise Lachapelle (Francia, 1769-1821), jefe de obstetricia en el Hôtel-Dieu de París, el hospital más antiguo de París. Publicó libros sobre la anatomía de la mujer, ginecología y obstetricia. Contraria al uso de fórceps, escribió Pratique des accouchements, y promovió los partos naturales. https://translate.google.es/translate?hl=es&sl=ca&u=https://ca.wikipedia.org/wiki/Marie-Louise_Lachapelle&prev=search Jane Haldiman Marcet (Londres, 1769-1858), divulgadora científica que escribió sobre química, enero 1 botánica, religión, economía y gramática. Publicó Conversations on Chemistry, con seudónimo masculino en 1805, pero no fue descubierta su autoría hasta 1837. https://lacienciaseacercaalcole.wordpress.com/2017/01/23/chicas-de-calendario-enero-primera-parte/ https://mujeresconciencia.com/2015/08/19/michael-faraday-y-jane-marcet-la-asimov-del-xix/ Montserrat Soliva Torrentó (Lérida, 1943-2019), doctora en ciencias químicas. https://es.wikipedia.org/wiki/Montserrat_Soliva_Torrent%C3%B3 Florence Lawrence (Canadá, 1886-1938), actriz del cine mudo apasionada por los coches, que inventó el intermitente, pero no lo consideró como propio y pasó el final de sus días sola y arruinada. https://www.motorpasion.com/espaciotoyota/el-dia-que-una-mujer-invento-el-intermitente-y-la-luz-de-freno-para-acabar-despues- arruinada Tewhida Ben Sheikh (Túnez, 1909-2010), primera mujer musulmana en convertirse en medica y llegó a plantear temas como la planificación familiar, la anticoncepción y el aborto en su época, en el norte enero 2 de Africa. -

SUMMARY of PERSONNEL ACTIONS REGENTS AGENDA June 2012
SUMMARY OF PERSONNEL ACTIONS REGENTS AGENDA June 2012 ANN ARBOR CAMPUS 1. Recommendations for approval of new appointments and promotions for regular associate and full professor ranks, with tenure. (1) Boehman, André L., professor of mechanical engineering, with tenure, College of Engineering, effective September 1, 2012. (2) Davenport, Christian, professor of political science, with tenure, College of Literature, Science, and the Arts, effective September 1, 2012. (3) Denton, Brian T., associate professor of industrial and operations engineering, with tenure, College of Engineering, effective September 1, 2012. (4) Fenstermaker, Sarah, professor of women’s studies, with tenure, College of Literature, Science, and the Arts, effective September 1, 2012. (5) Gold, David, associate professor of English language and literature, with tenure, College of Literature, Science, and the Arts, effective September 1, 2012. (6) Mihalcea, Rada F., associate professor of electrical engineering and computer science, with tenure, College of Engineering, effective September 1, 2013. (7) Neumar, Robert W., M.D., Ph.D., professor of emergency medicine, with tenure, effective July 1, 2012, and chair, Department of Emergency Medicine, Medical School, effective July 1, 2012 through June 30, 2017. (8) Ward, Brent B., promotion to associate professor of dentistry, with tenure, School of Dentistry, effective September 1, 2012 (currently assistant professor of dentistry.) 2. Recommendations for approval of new appointments and promotions for regular associate and full professor ranks, without tenure. (1) Abbott, Patricia, associate professor of nursing, without tenure, School of Nursing, effective September 1, 2012. 3. Recommendations for approval of reappointments of regular instructional staff and selected administrative/professional staff. (1) Appelman, Henry D., M.D., M.R. -

Read the Full PDF
The Science on Women and Science The Science on Women and Science Christina Hoff Sommers Editor The AEI Press Publisher for the American Enterprise Institute WASHINGTON, D.C. Distributed to the Trade by National Book Network, 15200 NBN Way, Blue Ridge Summit, PA 17214. To order call toll free 1-800-462-6420 or 1-717-794-3800. For all other inquiries please contact the AEI Press, 1150 Seventeenth Street, N.W., Washington, D.C. 20036 or call 1-800-862-5801. Library of Congress Cataloging-in-Publication Data The science on women and science / Christina Hoff Sommers, editor. p. cm. Includes bibliographical references. ISBN-13: 978-0-8447-4281-6 ISBN-10: 0-8447-4281-3 1. Women in science. 2. Sex discrimination against women. 3. Sex differences in education. I. Sommers, Christina Hoff. Q130.S364 2009 2009022004 13 12 11 10 09 1 2 3 4 5 © 2009 by the American Enterprise Institute for Public Policy Research, Washington, D.C. All rights reserved. No part of this publication may be used or reproduced in any manner whatsoever without permission in writing from the American Enterprise Institute except in the case of brief quotations embodied in news articles, critical articles, or reviews. The views expressed in the publications of the American Enterprise Institute are those of the authors and do not necessarily reflect the views of the staff, advisory panels, officers, or trustees of AEI. Printed in the United States of America Contents INTRODUCTION: THE SCIENCE ON WOMEN IN SCIENCE, Christina Hoff Sommers 1 Notes 5 References 6 1WHY SO FEW WOMEN IN MATH AND SCIENCE? Simon Baron-Cohen 7 Sex Differences in the General Population 11 Female Advantage in Empathy 15 Culture and Biology 18 Conclusions 18 Notes 20 References 21 2GENDER, MATH, AND SCIENCE, Elizabeth S. -
FALL2013.Pdf
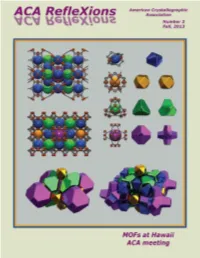
SC-XRD_ACA_PrintFP_D8 Quest ECO_Sep 2013 Print.pdf 1 9/3/13 1:22 PM American Crystallographic Association Cover: The cover image, from Omar Farha and Chris Wilmer, Northwestern University, depicts the metal-organic ACA RefleXions framework (MOF), of NU-111. Fall, 2013 See On the Cover, p 3. ACA HOME PAGE: www.AmerCrystalAssn.org Table of Contents 3 President’s Column, On the Cover, Sad News about Dave Rognlie The New D8 QUEST ECO 4 Letter to the Editor, Council Highlights, ACA RefleXions Co-Editors & Staff, Errata Crystallography with a Conscience 6 News & Awards 9 Introducing new staff member Chiara Pastore, 2013 Class of ACA Fellows 10 Contributors to this Issue 13 Net RefleXions, Index of Advertisers 15 Jerome Karle (1918 - 2013) 18 Ray Davis (1938 - 2013) C 20 Charles Norris Caughlan (1915 - 2013), Student Help in Hawaii M Y 21 Poster Prizes in Hawaii CM 24 Accompanying Members MY 25 Annual ACA Meeting in Hawaii CY 64 High School Outreach in Hawaii CMY 65 Books K 66 Workshop on Dynamic Structural Photocrystallography 67 Puzzle Corner 68 46th Course at Erice 71 Bruker/MIT Symposium 72 2014 ACA Meeting in Albuquerque 75 Corporate Members 77 Calendar of Meetings The D8 QUEST ECO Contributions to ACA RefleXions may be sent to either of the Editors: Please address matters pertaining to advertisements, membership With the D8 QUEST ECO, making the right choice is easy. Not only is it affordable and economical inquiries, or use of the ACA mailing list to: with minimal maintenance, it is also environmentally friendly offering low power requirements and Connie (Chidester) Rajnak Judith L. -

The Pursuit of Accurate Measurements: Gas Electron Diffraction from the 1930S to the 1960S
International Workshop on the History of Chemistry 2015 Tokyo The Pursuit of Accurate Measurements: Gas Electron Diffraction from the 1930s to the 1960s Mari Yamaguchi The University of Tokyo, Japan Introduction Most scientific instruments and experimental methods have been improved continually since their invention. Such improved methods sometimes change scientific practices and usually enable more accurate measurements that replace existing data with new ones. In his book, An Introduction to Scientific Research, the American chemist, E. Bright Wilson wrote the following about measurements at higher accuracy. Sometimes measurements at higher accuracy bring to light new and unforeseen discrepancies of fundamental importance. An example of this is the Lamb-Retherford measurements of hyperfine structure of hydrogen spectrum, which showed that the Dirac theory needed modification.1 This example showed the interrelationship between the theory and the measurement. The question this raises is: if there are discrepancies between the results of two measurement methods, does this render one of them obsolete or changed? To answer this question, I look to the case of the determination of molecular structures in the gas phase. Gas-phase molecules are less influenced by the environment than in the solid and liquid phase, and gas molecular structural studies have thus provided much fundamental information about molecular structures. In the 1960s, the methods for investigating gas molecules were gas electron diffraction and spectroscopy. In this paper, -

Chemical Knowledge in the Early Modern World'.', Osiris., 29 (1)
Durham Research Online Deposited in DRO: 25 March 2015 Version of attached le: Published Version Peer-review status of attached le: Peer-reviewed Citation for published item: Eddy, Matthew Daniel and Mauskopf, Seymour H. and Newman, William R. (2014) 'An introduction to 'Chemical knowledge in the early modern world'.', Osiris., 29 (1). pp. 1-15. Further information on publisher's website: http://dx.doi.org/10.1086/678110 Publisher's copyright statement: c 2014 by The History of Science Society. Additional information: Use policy The full-text may be used and/or reproduced, and given to third parties in any format or medium, without prior permission or charge, for personal research or study, educational, or not-for-prot purposes provided that: • a full bibliographic reference is made to the original source • a link is made to the metadata record in DRO • the full-text is not changed in any way The full-text must not be sold in any format or medium without the formal permission of the copyright holders. Please consult the full DRO policy for further details. Durham University Library, Stockton Road, Durham DH1 3LY, United Kingdom Tel : +44 (0)191 334 3042 | Fax : +44 (0)191 334 2971 https://dro.dur.ac.uk An Introduction to Chemical Knowledge in the Early Modern World by Matthew Daniel Eddy, Seymour H. Mauskopf, and William R. Newman THE SCOPE OF EARLY MODERN CHEMICAL KNOWLEDGE The essays in this volume collectively cover the development of chemistry in the “early modern world,” that is to say, from the ifteenth century through the eighteenth century.