View Our Culture Media & Consumables
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dehydrated Culture Media
Dehydrated Culture Media Manufactured by Dehydrated Culture Media Table of Contents 4 CRITERION™ Products 12 Supplements and Antibiotics 13 CRITERION™ Agarose for Gel Electrophoresis Dehydrated Culture Media ™ TM Hardy Diagnostics’ dehydrated culture media, CRITERION , is formulated to meet or exceed the highest quality standards. DEHYDRATED CULTURE MEDIA Choose from 250 standard formulas or request custom blending to your specifications. The innovative packaging designs and overall reliability makeCRITERION ™ the logical choice for culture media in your laboratory. FEATURES & BENEFITS Hand Grip Convenient hand-grip design features finger indentations to allow for easy and safe handling of the bottle. Induction Seal Gray Jar Pull-off induction seal prevents moisture from clumping the media, keeping it fresh and dry. Opaque gray jar diminishes Wide Mouth Opening light penetration, • Allows for easy access to use a scoop when prolonging superior measuring the powder. performance and • Prevents inhalation hazards and reduces shelf life. hazardous dust formations. • No more shaking the bottle to dispense the media. Desiccant Pack A silica gel pack is included in each bottle to prevent clumping. Reusable Seal A built-in cushion seal inside the lid prevents moisture from entering the previously opened container. 1 UNPARALLELED PERFORMANCE Every formulation and lot is thoroughly tested for optimal growth characteristics. WIDE MOUTH OPENING Scooping media from the wide mouth bottle, instead of pouring and shaking, reduces dangerous dust formation CONVENIENT SIZES Packaged in four standard sizes to fit your needs: • 2 liter Mylar® bag (pre-measured to make 2 liters of culture media) • 500gm bottle • 2kg buckets with locking screw top lid • 10kg buckets with locking screw top lid STACKABLE Bottles and buckets have a nesting design and are stackable for efficient and economical storage. -

Reverse Blot Hybridization Assay
Detection of Waterborne Pathogens by Polymerase Chain Reaction- Reverse Blot Hybridization Assay Yeonim Choi The Graduate School Yonsei University Department of Biomedical Laboratory Science Detection of Waterborne Pathogens by Polymerase Chain Reaction- Reverse Blot Hybridization Assay A Dissertation Submitted to the Department of Biomedical Laboratory Science and the Graduate School of Yonsei University in partial fulfillment of the requirements for the degree of Doctor of Philosophy Yeonim Choi July 2011 G This certifies that the dissertation of Yeonim Choi is approved. Thesis Supervisor : Hyeyoung Lee Ok Doo Awh : Thesis Committee Member Tae Ue Kim : Thesis Committee Member Jong Bae Kim : Thesis Committee Member Yong Serk Park : Thesis Committee Member The Graduate School Yonsei University July 2011 G G Dedicated to my family and my friends, who have encouraged me. G G CONTENTS LIST OF FIGURES AND TABLES ------------------------------------------------ iv ABBREVIATIONS ------------------------------------------------------------------- ix ABSTRACT IN ENGLISH ----------------------------------------------------------- x I. INTRODUCTION ------------------------------------------------------------- 1 II. MATERIALS AND METHODS -------------------------------------------- 9 1. Development of PCR-REBA targeting waterborne pathogens -------- 9 Bacterial reference strains and cultivation ------------------------------- 9 Genomic DNA extraction -

Pocket Guide to Clinical Microbiology
4TH EDITION Pocket Guide to Clinical Microbiology Christopher D. Doern 4TH EDITION POCKET GUIDE TO Clinical Microbiology 4TH EDITION POCKET GUIDE TO Clinical Microbiology Christopher D. Doern, PhD, D(ABMM) Assistant Professor, Pathology Director of Clinical Microbiology Virginia Commonwealth University Health System Medical College of Virginia Campus Washington, DC Copyright © 2018 Amer i can Society for Microbiology. All rights re served. No part of this publi ca tion may be re pro duced or trans mit ted in whole or in part or re used in any form or by any means, elec tronic or me chan i cal, in clud ing pho to copy ing and re cord ing, or by any in for ma tion stor age and re trieval sys tem, with out per mis sion in writ ing from the pub lish er. Disclaimer: To the best of the pub lish er’s knowl edge, this pub li ca tion pro vi des in for ma tion con cern ing the sub ject mat ter cov ered that is ac cu rate as of the date of pub li ca tion. The pub lisher is not pro vid ing le gal, med i cal, or other pro fes sional ser vices. Any ref er ence herein to any spe cific com mer cial prod ucts, pro ce dures, or ser vices by trade name, trade mark, man u fac turer, or oth er wise does not con sti tute or im ply en dorse ment, rec om men da tion, or fa vored sta tus by the Ameri can Society for Microbiology (ASM). -

Antimicrobial Resistance EMERGING INFECTIOUS DISEASES Pages 681-814 Peer-Reviewed Journal Tracking and Analyzing Disease Trends Pages 681–814
Vol 13, No 5, May 2007 Vol ® May 2007 Antimicrobial Resistance EMERGING INFECTIOUS DISEASES Pages 681-814 Pages Peer-Reviewed Journal Tracking and Analyzing Disease Trends pages 681–814 EDITOR-IN-CHIEF D. Peter Drotman EDITORIAL STAFF EDITORIAL BOARD Managing Senior Editor Dennis Alexander, Addlestone Surrey, United Kingdom Polyxeni Potter, Atlanta, Georgia, USA Barry J. Beaty, Ft. Collins, Colorado, USA Associate Editors Martin J. Blaser, New York, New York, USA Paul Arguin, Atlanta, Georgia, USA David Brandling-Bennet, Washington, D.C., USA Charles Ben Beard, Ft. Collins, Colorado, USA Donald S. Burke, Baltimore, Maryland, USA David Bell, Atlanta, Georgia, USA Arturo Casadevall, New York, New York, USA Jay C. Butler, Anchorage, Alaska, USA Kenneth C. Castro, Atlanta, Georgia, USA Charles H. Calisher, Ft. Collins, Colorado, USA Thomas Cleary, Houston, Texas, USA Stephanie James, Bethesda, Maryland, USA Anne DeGroot, Providence, Rhode Island, USA Brian W.J. Mahy, Atlanta, Georgia, USA Vincent Deubel, Shanghai, China Paul V. Effler, Honolulu, Hawaii, USA Nina Marano, Atlanta, Georgia, USA Ed Eitzen, Washington, D.C., USA Martin I. Meltzer, Atlanta, Georgia, USA Duane J. Gubler, Honolulu, Hawaii, USA David Morens, Bethesda, Maryland, USA Richard L. Guerrant, Charlottesville, Virginia, USA J. Glenn Morris, Baltimore, Maryland, USA Scott Halstead, Arlington, Virginia, USA Marguerite Pappaioanou, St. Paul, Minnesota, USA David L. Heymann, Geneva, Switzerland Tanja Popovic, Atlanta, Georgia, USA Daniel B. Jernigan, Atlanta, Georgia, USA Patricia M. Quinlisk, Des Moines, Iowa, USA Charles King, Cleveland, Ohio, USA Jocelyn A. Rankin, Atlanta, Georgia, USA Keith Klugman, Atlanta, Georgia, USA Didier Raoult, Marseilles, France Takeshi Kurata, Tokyo, Japan Pierre Rollin, Atlanta, Georgia, USA S.K. -

CTA with Carbohydrates Is a Semi-Solid Medium Suitable for the Determination of Fermentation Reactions of Fastidious Microorganisms
Administrative Offices Phone: 207-873-7711 Fax: 207-873-7022 Customer Service Phone: 1-800-244-8378 P.O. Box 788 Fax: 207-873-7022 Waterville, Maine 04903-0788 RT. 137, China Road Winslow, Maine 04901 TECHNICAL PRODUCT INFORMATION CYSTINE TRYPTIC AGAR [CTA] w/ or w/o CARBOHYDRATES Catalog No: T1400 Control (w/o Carbohydrates) T1410 CTA w/DEXTROSE T1440 CTA w/MALTOSE T1420 CTA w/FRUCTOSE T1445 CTA w/MANNITOL T1430 CTA w/LACTOSE T1450 CTA w/SUCROSE T1435 CTA w/XYLOSE T0340 CTA w/SORBOSE T0350 CTA w/INULIN T0355 CTA w/SORBITOL INTENDED USE: CTA with carbohydrates is a semi-solid medium suitable for the determination of fermentation reactions of fastidious microorganisms. CTA medium without carbohydrates is suitable for maintenance of organisms, and for detection of motility. HISTORY/SUMMARY: CTA medium has been accepted for the determination of carbohydrate utilization for a number of fastidious organisms, particularly Neisseria species and anaerobes. It has also been reported useful in fermentation studies of yeast. As a maintenance medium without carbohydrates, it supports the growth of organisms such as Neisseria, Pasteurella, Streptococci, Brucella, Corynebacteria and others. Motility can be detected in the semisolid medium when inoculated by stab line. PRINCIPLES: The base medium is free of carbohydrates and meat extracts. It contains Cystine and Casein Peptone as nutrients for the growth of fastidious organisms. Phenol red is added as an indicator of fermentation reactions. Carbohydrates are usually incorporated in the medium in 1% final concentrations. If a microorganism is inoculated in the medium containing a carbohydrate, and is capable of fermenting it, the medium indicator will turn from orange red to yellow. -

Product List 2008/09
Product List 2008/09 Part of Thermo Fisher Scientific Oxoid Chromogenic Media … Sheer Brilliance™ to reflect this, the names of products are being changed so that the current range now includes: Brilliance Bacillus cereus Agar Brilliance Candida Agar Brilliance E. coli/coliform Agar Brilliance E. coli/coliform Selective Agar Brilliance Enterobacter sakazakii Agar (DFI) Brilliance Listeria Agar Brilliance UTI Agar Brilliance UTI Clarity Agar Brilliance Salmonella Agar The chromogens used in our Brilliance range of chromogenic media ensure that colony colours are vivid, allowing rapid, easy differentiation and presumptive identification of the organism in question. So remember Brilliance – chromogenic media that delivers clearly visible answers on a single culture plate. Contents 1 Culture Media & Supplements 2 Supplementary Reagents 20 Antibiotic Single Supplements 21 Biochemical Reagents 22 Laboratory Preparations & Biological Extracts 22 Veggietones 24 Bagged Media 25 Dip Slides 25 Atmosphere Generation 26 AGS 26 Traditional Systems 27 Food Testing 28 DuPont Qualicon 29 BAX® System, Test and Components 29 Culture Media for the BAX System 30 RiboPrinter® Microbial Characterisation System 31 Lateral Flow System Range 31 Blood Culture 32 Signal 32 Wampole Isolator® 32 Antimicrobial Susceptibility Testing 33 M.I.C.Evaluator Strips 33 aura image 34 AST Accessories 34 Antimicrobial Susceptibility Testing Discs 35 Miscellaneous 38 Sundries 38 Publications 38 Diagnostics 39 Biochemical I.D. 39 Toxin Detection Kits 40 Agglutination Tests 41 Enzyme Immunoassays 42 Immunofluorescence Assays 43 Lateral Flow Assays 44 Environmental Monitoring and Process Simulation 45 Oxoid Air Sampler 45 Environmental Monitoring Swabs 45 Prepared Media 45 Dehydrated Media 45 Process Simulation 46 2 Culture Media and Supplements A wide range of general purpose, enrichment, selective and differential Anaerobe Basal Broth media, diluents and supplements a medium for general growth of anaerobes NB: information provided here is intended only as an aid to purchasing. -

The Cultivable Autochthonous Microbiota of the Critically Endangered Northern Bald Ibis (Geronticus Eremita)
RESEARCH ARTICLE The cultivable autochthonous microbiota of the critically endangered Northern bald ibis (Geronticus eremita) Joachim Spergser1*, Igor Loncaric1, Alexander Tichy2, Johannes Fritz3, Alexandra Scope4 1 Institute of Microbiology, Department of Pathobiology, University of Veterinary Medicine, Vienna, Austria, 2 Bioinformatics and Biostatistics Platform, Department of Biomedical Sciences, University of Veterinary Medicine, Vienna, Austria, 3 Waldrappteam, Mutters, Austria, 4 Clinical Unit of Internal Medicine Small a1111111111 Animals, Department/Clinic for Companion Animals and Horses, University of Veterinary Medicine, Vienna, a1111111111 Austria a1111111111 a1111111111 * [email protected] a1111111111 Abstract The critically endangered Northern bald ibis (Geronticus eremita) is a migratory bird that OPEN ACCESS became extinct in Europe centuries ago. Since 2014, the Northern bald ibis is subject to an Citation: Spergser J, Loncaric I, Tichy A, Fritz J, intensive rehabilitation and conservation regime aiming to reintroduce the bird in its original Scope A (2018) The cultivable autochthonous distribution range in Central Europe and concurrently to maintain bird health and increase microbiota of the critically endangered Northern bald ibis (Geronticus eremita). PLoS ONE 13(4): population size. Hitherto, virtually nothing is known about the microbial communities associ- e0195255. https://doi.org/10.1371/journal. ated with the ibis species; an information pivotal for the veterinary management of these pone.0195255 birds. Hence, the present study was conducted to provide a baseline description of the culti- Editor: Michael Lierz, Justus-Liebeig University vable microbiota residing in the Northern bald ibis. Samples derived from the choana, tra- Giessen, GERMANY chea, crop and cloaca were examined employing a culturomic approach in order to identify Received: June 30, 2017 microbes at each sampling site and to compare their frequency among age classes, sea- Accepted: March 19, 2018 sonal appearances and rearing types. -

Prepared Culture Media
PREPARED CULTURE MEDIA 121517SS PREPARED CULTURE MEDIA Made in the USA AnaeroGRO™ DuoPak A 02 Bovine Blood Agar, 5%, with Esculin 13 AnaeroGRO™ DuoPak B 02 Bovine Blood Agar, 5%, with Esculin/ AnaeroGRO™ BBE Agar 03 MacConkey Biplate 13 AnaeroGRO™ BBE/PEA 03 Bovine Selective Strep Agar 13 AnaeroGRO™ Brucella Agar 03 Brucella Agar with 5% Sheep Blood, Hemin, AnaeroGRO™ Campylobacter and Vitamin K 13 Selective Agar 03 Brucella Broth with 15% Glycerol 13 AnaeroGRO™ CCFA 03 Brucella with H and K/LKV Biplate 14 AnaeroGRO™ Egg Yolk Agar, Modified 03 Buffered Peptone Water 14 AnaeroGRO™ LKV Agar 03 Buffered Peptone Water with 1% AnaeroGRO™ PEA 03 Tween® 20 14 AnaeroGRO™ MultiPak A 04 Buffered NaCl Peptone EP, USP 14 AnaeroGRO™ MultiPak B 04 Butterfield’s Phosphate Buffer 14 AnaeroGRO™ Chopped Meat Broth 05 Campy Cefex Agar, Modified 14 AnaeroGRO™ Chopped Meat Campy CVA Agar 14 Carbohydrate Broth 05 Campy FDA Agar 14 AnaeroGRO™ Chopped Meat Campy, Blood Free, Karmali Agar 14 Glucose Broth 05 Cetrimide Select Agar, USP 14 AnaeroGRO™ Thioglycollate with Hemin and CET/MAC/VJ Triplate 14 Vitamin K (H and K), without Indicator 05 CGB Agar for Cryptococcus 14 Anaerobic PEA 08 Chocolate Agar 15 Baird-Parker Agar 08 Chocolate/Martin Lewis with Barney Miller Medium 08 Lincomycin Biplate 15 BBE Agar 08 CompactDry™ SL 16 BBE Agar/PEA Agar 08 CompactDry™ LS 16 BBE/LKV Biplate 09 CompactDry™ TC 17 BCSA 09 CompactDry™ EC 17 BCYE Agar 09 CompactDry™ YMR 17 BCYE Selective Agar with CAV 09 CompactDry™ ETB 17 BCYE Selective Agar with CCVC 09 CompactDry™ YM 17 BCYE -

APPENDIX a Media and Reagents
APPENDIX A Media and Reagents Pauline K. w. Yu, M.S. The use of appropriate and dependable media is integral to the isolation and identification of microorganisms. Unfortunately, comparative data docu menting the relative efficacy or value of media designed for similar purposes are often lacking. Moreover, one cannot presume identity in composition of a given generic product which is manufactured by several companies because each may supplement the generic products with components, often of a proprietary nature and not specified in the product's labeling. Finally, the actual production of similar products may vary among manufacturers to a sufficient extent to affect their performance. For all of these reasons, therefore, product selection for the laboratory should not be strictly based on cost considerations and should certainly not be based on promotional materials. Evaluations that have been published in the scientific literature should be consulted when available. Alternatively, the prospective buyer should consult a recognized authority in the field. It is seldom necessary for the laboratory to prepare media using basic components since these are usually available combined in dehydrated form from commercial sources; however, knowledge of a medium's basic compo nents is helpful in understanding how the medium works and what might be wrong when it does not work. Hence, the components have been listed for each medium included in this chapter. All dehydrated media must be prepared exactly according to the manu facturers' directions. Any deviation from these directions may adversely affect or significantly alter a medium's performance. Containers of media should be dated on receipt and when opened, and the media should never be used beyond expiration dates specified by the manufacturers or recom mended by quality control programs. -

View Our Full Water Sampling Vials Product Offering
TABLE OF CONTENTS Environmental Monitoring 1 Sample Prep and Dilution 8 Dehydrated Culture Media - Criterion™ 12 Prepared Culture Media 14 Chromogenic Media - HardyCHROM™ 18 CompactDry™ 20 Quality Control 24 Membrane Filtration 25 Rapid Tests 26 Lab Supplies/Sample Collection 27 Made in the USA Headquarters Distribution Centers 1430 West McCoy Lane Santa Maria, California Santa Maria, CA 93455 Olympia, Washington 800.266.2222 : phone Salt Lake City, Utah [email protected] Phoenix, Arizona HardyDiagnostics.com Dallas, Texas Springboro, Ohio Hardy Diagnostics has a Quality Lake City, Florida Management System that is certified Albany, New York to ISO 13485 and is a FDA licensed Copyright © 2020 Hardy Diagnostics Raleigh, North Carolina medical device manufacturer. Environmental Monitoring Hardy Diagnostics offers a wide selection of quality products intended to help keep you up to date with regulatory compliance, ensure the efficacy of your cleaning protocol, and properly monitor your environment. 800.266.2222 [email protected] HardyDiagnostics.com 1 Environmental Monitoring Air Sampling TRIO.BAS™ Impact Air Samplers introduced the newest generation of microbial air sampling. These ergonomically designed instruments combine precise air sampling with modern connectivity to help you properly assess the air quality of your laboratory and simplify your process. MONO DUO Each kit includes: Each kit includes: TRIO.BAS™ MONO air sampler, induction TRIO.BAS™ DUO air sampler, battery battery charger and cable, aspirating charger -

WHO Global Foodborne Infections Network
WHO Global Foodborne Infections Network (formerly WHO Global Salm-Surv) "A WHO network building capacity to detect, control and prevent foodborne and other enteric infections from farm to table” Laboratory Protocol “Isolation of Salmonella spp. From Food and Animal Faeces ” 5th Ed. June 2010 1 IMPORTANT NOTES: 1) This procedure is based on the ISO protocol: 6579:2002 “Microbiology of food and animal feeding stuffs -- Horizontal method for the detection of Salmonella spp.”4. This protocol is intended to provide guidance for the testing of suspect food items/ animal faecal specimens identified via foodborne disease surveillance programmes. Regulatory agencies (Ministries of Health, Agriculture, Commerce, etc) have specific testing requirements, different from this protocol, which much be used to test samples collected for regulatory testing (example: import/export or product recall). Prior to performing any official, legal, or regulatory testing, the reader should confirm the appropriate protocol through consult with in-country regulatory authorities. 2) This protocol is intended only to be used on food samples and animal faeces. This protocol should not be used for the testing of human faeces. Foreword: Infections due to Salmonella spp. remain a global problem. These infections may cause significant morbidity and mortality both in humans and production animals as well as considerable economic losses. Salmonella spp. are typically transmitted among humans and animals via a fecal-oral route, usually through the consumption of contaminated food or water. Timely identification and serotyping of Salmonella from clinical specimens facilitates outbreak detection and patient management while prompt and accurate detection of Salmonella spp. in contaminated food or water provides an opportunity to prevent the contaminated food from entering the food supply. -
Nutrient Broth (N7519)
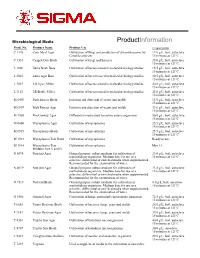
Microbiological Media ProductInformation Prod. No. Product Name Product Use Preparation C 1176 Corn Meal Agar Cultivation of fungi and production of chlamydospores by 17.0 g/L, boil, autoclave Candida albicans 15 minutes at 121ºC. C 1551 Czapek Dox Broth Cultivation of fungi and bacteria 35.0 g/L, boil, autoclave 15 minutes at 121ºC. L 1900 Luria Broth Base Cultivation of bacteria used in molecular biology studies. 15.5 g/L, boil, autoclave 15 minutes at 121ºC. L 2025 Luria Agar Base Cultivation of bacteria used in molecular biology studies. 30.5 g/L, boil, autoclave 15 minutes at 121ºC. L 3027 LB Agar, Miller Cultivation of bacteria used in molecular biology studies. 40.0 g/L; boil, autoclave 15 minutes at 121ºC. L 3152 LB Broth, Miller Cultivation of bacteria used in molecular biology studies. 25.0 g/L; boil, autoclave 15 minutes at 121ºC. M 6409 Malt Extract Broth Isolation and detection of yeasts and molds 15.0 g/L, boil, autoclave 15 minutes at 121ºC. M 6907 Malt Extract Agar Isolation and detection of yeasts and molds 33.6 g/L, boil, autoclave 15 minutes at 121ºC. M 7408 MacConkey Agar Differential media used to isolate enteric organisms 50.0 g/L; boil, autoclave 15 minutes at 121ºC M 0660 Mycoplasma Agar Cultivation of mycoplasma 35.5 g/L; boil, autoclave 15 minutes at 121ºC M 0535 Mycoplasma Broth Cultivation of mycoplasma 25.5 g/L; boil, autoclave 15 minutes at 121ºC M 1539 Mycoplasma Test Broth Cultivation of mycoplasma Ready to use. M 1914 Mycoplasma Test Cultivation of mycoplasma Mix 1:1.