Cone Interaction with Progressive Macular Dysfunction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Congenital Ocular Anomalies in Newborns: a Practical Atlas
www.jpnim.com Open Access eISSN: 2281-0692 Journal of Pediatric and Neonatal Individualized Medicine 2020;9(2):e090207 doi: 10.7363/090207 Received: 2019 Jul 19; revised: 2019 Jul 23; accepted: 2019 Jul 24; published online: 2020 Sept 04 Mini Atlas Congenital ocular anomalies in newborns: a practical atlas Federico Mecarini1, Vassilios Fanos1,2, Giangiorgio Crisponi1 1Neonatal Intensive Care Unit, Azienda Ospedaliero-Universitaria Cagliari, University of Cagliari, Cagliari, Italy 2Department of Surgery, University of Cagliari, Cagliari, Italy Abstract All newborns should be examined for ocular structural abnormalities, an essential part of the newborn assessment. Early detection of congenital ocular disorders is important to begin appropriate medical or surgical therapy and to prevent visual problems and blindness, which could deeply affect a child’s life. The present review aims to describe the main congenital ocular anomalies in newborns and provide images in order to help the physician in current clinical practice. Keywords Congenital ocular anomalies, newborn, anophthalmia, microphthalmia, aniridia, iris coloboma, glaucoma, blepharoptosis, epibulbar dermoids, eyelid haemangioma, hypertelorism, hypotelorism, ankyloblepharon filiforme adnatum, dacryocystitis, dacryostenosis, blepharophimosis, chemosis, blue sclera, corneal opacity. Corresponding author Federico Mecarini, MD, Neonatal Intensive Care Unit, Azienda Ospedaliero-Universitaria Cagliari, University of Cagliari, Cagliari, Italy; tel.: (+39) 3298343193; e-mail: [email protected]. -

Ocular Emergencies for the Primary Care Optometrist
Ocular Emergencies Ocular Emergencies for the Disclosure Statement Primary Care Optometrist . Honorarium, Speaker, Consultant, Research Grant: Aerie, Alcon, Allergan, B+L, Carl Zeiss, Glaukos, Heidelberg, Novartis, Topcon, Michael Chaglasian, OD, FAAO Associate Professor Illinois Eye Institute Illinois College of Optometry [email protected] What is a “True” Emergency? “True” Emergency . Pain (vs. discomfort) . History is key to differentiating emergency versus urgency . Current or potential for: Phone or in person Vision loss Proper triage is essential Structural damage After hours protocol Needs immediate (same day) attention Your office and your specialists Medico-legal implications History Emergency Exam Vision Recent ocular disease or One or both eyes? surgery . Acuity . External examination Visual field Other diseases . Visual fields . SLE Sudden or gradual cardiac, vascular, or . Pupils Blurred or lost? autoimmune . IOP Diplopia? viruses . Ocular Motility . Fundus exam Mono or Bino Medications or recent Pain changes to medications Redness Nausea/vomiting Onset Trauma Contact lenses M. Chaglasian, OD 1 Ocular Emergencies Emergency Kit “True” Emergency . Chemical Burns . Eye shield . pH paper Alkaline . Pressure patch . Bandage CL’s . Sterile eye wash . Diamox . Central Retinal Artery Occlusion . Alger brush . Topical drops . Forceps Antibiotics NSAID’s . Golf spud . Both have extremely high risk of severe and permanent Steroids vision loss which can be prevented via immediate Cycloplegics intervention and treatment Chemical Trauma Chemical Burns . Copious irrigation anesthetic . Acid exposure speculum Only penetrate through epithelium sterile saline v tap water car battery, vinegar, and some refrigerants . Contacts can be removed after irrigation . Sweep fornices – repeatedly . Alkaline exposure Penetrates tissues more easily and . Examination after irrigation and neutralization of pH have a prolonged effect . -

The EEC Syndrome and Its Ocular Manifestations
Br J Ophthalmol: first published as 10.1136/bjo.73.4.261 on 1 April 1989. Downloaded from British Journal ofOphthalmology, 1989, 73, 261-264 The EEC syndrome and its ocular manifestations ALAN A McNAB,* MICHAEL J POTTS, AND RICHARD A N WELHAM From the Lacrimal Clinic, Moorfields Eye Hospital, London, EC] V2PD SUMMARY The EEC syndrome (ectrodactyly or lobster-claw deformity, ectodermal dysplasia, and cleft lip and palate) is a rare disorder with autosomal dominant inheritance, variable expression, and in some families lack of penetrance. We present the findings in five cases with emphasis on the ocular findings. Lacrimal surgery was performed on three patients with good results in each case. We also report the occurrence of spontaneous corneal perforation in two cases, a complication not previously recognised. The ophthalmic care of these patients must be pursued long-term, as progressive visual impairment may be the most disabling feature of the syndrome. The EEC syndrome, and acronym coined by Rudiger provide a more functional hand. Both feet had et al in 1970,' was first recognised as a distinct clinical syndactyly. Her hair was coarse and dry and her skin entity by Cockayne.2 He drew attention to the subject to eczema. She had a bilateral conductive dacryocystitis which commonly afflicts these patients, hearing loss and wore hearing aids. There was and found that it was associated with 'atresia of the bilateral malar hypoplasia and small malformed lacrimal ducts.' Since that description in 1936, several teeth. other reports have appeared in the ophthalmic We first saw her at the age of 11 years. -

Obstructed Tear Duct Causes Epiphora and Precocious Eyelid Opening Due
bioRxiv preprint doi: https://doi.org/10.1101/2020.04.17.046383; this version posted April 17, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Obstructed tear duct causes epiphora and precocious eyelid opening due to disruption of Prickle 1-mediated Wnt/PCP signaling Dianlei Guo1*, Jiali Ru1*, Jiaying Fan2*, Rong Ju1, Kangxin Jin1, Hong Ouyang1, Lai Wei1, Yizhi Liu1, Chunqiao Liu1$ 1, State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, Sun Yat-sen University, Guangzhou 510060, China 2, Guangzhou Woman & Children’s Medical Center *Equal contribution $Correspondence should be addressed to Dr. Chunqiao Liu: Email: [email protected] bioRxiv preprint doi: https://doi.org/10.1101/2020.04.17.046383; this version posted April 17, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Abstract The tear drainage apparatus evolved in terrestrial animals serving as conduits for tear flow. Obstruction of tear drainage causes a range of ocular surface disorders. Hitherto, genetics of tear duct development and obstruction has been scarcely explored. Here we report that a severe Prickle 1 hypomorph mouse line exhibited epiphora. This phenotype was due to blockage of the tear drainage by the incompletely formed nasolacrimal duct (NLD) and lacrimal canaliculi (CL). -

Acquired Etiologies of Lacrimal System Obstructions
5 Acquired Etiologies of Lacrimal System Obstructions Daniel P. Schaefer Acquired obstructions of the lacrimal excretory outfl ow system will produce the symptoms of epiphora, mucopurulent discharge, pain, dacryocystitis, and even cellulitis, prompting the patient to seek the ophthalmologist for evaluation and treatment. Impaired tear outfl ow may be functional, structural, or both. The causes may be primary – those resulting from infl ammation of unknown causes that lead to occlusive fi brosis—or secondary, resulting from infections, infl amma- tion, trauma, malignancies, toxicity, or mechanical causes. Secondary acquired dacryostenosis and obstruction may result from many causes, both common and obscure. Occasionally, the precise pathogenesis of nasolacrimal duct obstruction will, despite years of investigations, be elusive. To properly evaluate and appropriately treat the patient, the ophthal- mologist must have knowledge and comprehension of the lacrimal anatomy, the lacrimal apparatus, pathophysiology, ocular and nasal relationships, ophthalmic and systemic disease process, as well as the topical and systemic medications that can affect the nasolacrimal duct system. One must be able to assess if the cause is secondary to outfl ow anomalies, hypersecretion or refl ex secretion, pseudoepiphora, eyelid malposition abnormalities, trichiasis, foreign bodies and conjunctival concretions, keratitis, tear fi lm defi ciencies or instability, dry eye syn- dromes, ocular surface abnormalities, irritation or tumors affecting the trigeminal nerve, allergy, medications, or environmental factors. Abnormalities of the lacrimal pump function can result from involu- tional changes, eyelid laxity, facial nerve paralysis, or fl oppy eyelid syndrome, all of which displace the punctum from the lacrimal lake. If the cause is secondary to obstruction of the nasolacrimal duct system, the ophthalmologist must be able to determine where the anomaly is and what the cause is, in order to provide the best treatment possible for the patient. -

Exotic Animal Ophthalmology
Exotic Animal Ophthalmology University of Florida Tilapia fish Cataracts Fish Eye Salmon in Norway Goldfish cataract Rabbits Single Punctum Glaucoma Normal Indolent ulcers in dwarf rabbits Orbital abscess Warbles Iris granulomas: Not Pasteurella but lens capsule rupture from protozoan Encephalitozoa cuniculi Corneal edema Thick cornea Conjunctival overgrowth Guinea pig with corneal dystrophy Bone Guinea pig with bone in angle Guinea pig with corneal ulcer Parasitic blepharitis Trixacurus Guinea pig fundus Chinchilla KCS Gerbil with cherry eye Ferret and tonometry (IOP= 18-28 mmHg) Ferret cataract Ferret Rats Cataract and uveitis SDA: Coronavirus Mouse with keratitis Hamster with plasma cell conjunctivitis/blepharitis Birds X MRI Cockatiel with ankyloblepharon TE adhered Exophthalmos Glaucoma Chlamydial conjunctivitis Hibernoma of TE Eye flukes: Philophthalmus Conjunctivitis from Philophthalmus in a Capybara Eagle with pellet in eye ICA loosely attached in birds Angle Penetrating corneal ulcer Pecten Pecten Optic Nerve Cormorant Comorant Fovea Filoplumes Scleral ossicles Endophthalmitis Air bubbles indicate ossicle rupture Cataracts Lens luxation X Cavum lenticuli Retinal degeneration Giraffe Elephants Big Cats Cougar Lion with FCRD Tiger ERG Pilot whale Total tapetum Fruit bat retina Fruit bat X Choroidal papillae provide retinal nutrition Ulcer Marsupials Cataracts Wallaby Exotropic Possum Reptiles/Amphibians Camouflage Gecko pupil Fire bellied toads with uveitis Fibropapilloma of green turtles Enucleated African frogs can eat Lipid keratopathy in tree frogs Males have larger eyes in this Texas rat snake Spectacles are fused lids Retained spectacle After molting Subspectacular abscess Oral infections (Aeromonas) Hypovitaminosis A ????????? ???? GO GATORS!!! Hypopyon: uveitis . -

AFFECTIONS of EYELIDS in ANIMALS Affections of Eyelids in Animals
AFFECTIONS OF EYELIDS IN ANIMALS Affections of eyelids in animals Anatomy and physiology; • Consists of movable folds of skin, loose connective tissue, muscular tissue, tarsus and conjunctiva. • Dorsal and ventral folds form palpebral fissure. • Sensory supply is by ophthalmic to upper eyelid and maxillary to the lower eyelid. • The upper eyelid is more movable. • Have protective eyelashes (cilia) at the margins • Upper and lower eyelids unite to form lateral and median canthi. • There is a well developed orbicularis oculi muscle. Sphincter muscle responsible for blinking. Innervated by palpebral branch of facial nerve. • Posterior to muscle is dense connective tissue layer called tarsus. A poorly defined fibrous sheet (well developed in upper eyelid) which continues with septum orbitale. • Dorsal to tarsus is levator palpebrae superiosis which helps to raise the upper eyelid. Innervated by oculomotor nerve. • Deep to levator is Miller’s muscle which is innervated by sympathetics. Paralysis of this muscle causes a disease called Ptosis i.e. drooping of upper eyelid (Horner’s syndrome). • Contains meibomian glands or tarsal glands having outlets as tiny apertures along the lid margins. • The innermost layer of eyelid is palpebral conjunctiva containing lymphoid follicles, accessory lacrimal glands of Krause and Wolfring. Blinking: • 90% bilateral in human beings. • 80% in dogs. • 60% in cattle and other animals. • Voluntary due to trauma. • Involuntary, normally 25/5min in all. May go as high as 100/5min. • Helps in continuous spreading of precorneal tear film over cornea. • Helps in continuous removal of precorneal tear film towards median canthus. • Helps in the removal of foreign bodies, if any. -
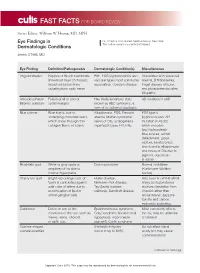
Eye Findings in Dermatologic Conditions
FAST FACTS FOR BOARD REVIEW Series Editor: William W. Huang, MD, MPH Eye Findings in Dr. O’Neill is from Buffalo Medical Group, New York. The author reports no conflict of interest. Dermatologic Conditions Jenna O’Neill, MD Eye Finding Definition/Pathogenesis Dermatologic Condition(s) Miscellaneous Angioid streaks Rupture of Bruch membrane PXE, EDS (kyphoscoliosis and Associated with sickle cell (innermost layer of choroid); vascular types most commonly anemia, β thalassemia, broad red-brown lines associated), Cowden disease Paget disease of bone, radiating from optic nerve and phosphatemia; often idiopathic Ankyloblepharon Fusion of all or part of Hay-Wells syndrome (also AD mutation in p63 filiforme adnatum eyelid margins known as AEC syndrome, a form of ectodermal dysplasia) Blue sclerae Blue hue is due to Alkaptonuria, EDS, Fanconi EDS type 6 underlying choroidal veins, anemia, Marfan syndrome, (kyphoscoliosis; AR which show through thin nevus of Ota, osteogenesis mutation in PLOD, collagen fibers of sclera imperfecta types I–III, PXE which encodes lysyl hydroxylase): blue sclerae, retinal detachment, globe rupture, keratoconus; also found in alkaptonuria and nevus of Ota due to pigment deposition in sclera Brushfield spot White to gray spots at Down syndrome Normal in children periphery of iris due to (Kunkmann-Wolffian stromal hyperplasia bodies) Cherry red spot Bright red-orange color of Hurler disease, Also seen in central retinal fovea is contrasted against Niemann-Pick disease, artery occlusion (fovea pale color of retina due -

EUROCAT Syndrome Guide
JRC - Central Registry european surveillance of congenital anomalies EUROCAT Syndrome Guide Definition and Coding of Syndromes Version July 2017 Revised in 2016 by Ingeborg Barisic, approved by the Coding & Classification Committee in 2017: Ester Garne, Diana Wellesley, David Tucker, Jorieke Bergman and Ingeborg Barisic Revised 2008 by Ingeborg Barisic, Helen Dolk and Ester Garne and discussed and approved by the Coding & Classification Committee 2008: Elisa Calzolari, Diana Wellesley, David Tucker, Ingeborg Barisic, Ester Garne The list of syndromes contained in the previous EUROCAT “Guide to the Coding of Eponyms and Syndromes” (Josephine Weatherall, 1979) was revised by Ingeborg Barisic, Helen Dolk, Ester Garne, Claude Stoll and Diana Wellesley at a meeting in London in November 2003. Approved by the members EUROCAT Coding & Classification Committee 2004: Ingeborg Barisic, Elisa Calzolari, Ester Garne, Annukka Ritvanen, Claude Stoll, Diana Wellesley 1 TABLE OF CONTENTS Introduction and Definitions 6 Coding Notes and Explanation of Guide 10 List of conditions to be coded in the syndrome field 13 List of conditions which should not be coded as syndromes 14 Syndromes – monogenic or unknown etiology Aarskog syndrome 18 Acrocephalopolysyndactyly (all types) 19 Alagille syndrome 20 Alport syndrome 21 Angelman syndrome 22 Aniridia-Wilms tumor syndrome, WAGR 23 Apert syndrome 24 Bardet-Biedl syndrome 25 Beckwith-Wiedemann syndrome (EMG syndrome) 26 Blepharophimosis-ptosis syndrome 28 Branchiootorenal syndrome (Melnick-Fraser syndrome) 29 CHARGE -

Meeting Materials
BUSINESS, CONSUMER SERVICES, AND HOUSING AGENCY EDMUND G. BROWN JR., GOVERNOR STATE BOARD OF OPTOMETRY 2450 DEL PASO ROAD, SUITE 105, SACRAMENTO, CA 95834 P (916) 575-7170 F (916) 575-7292 www.optometry .ca.gov Continuing Education Course Approval Checklist Title: Provider Name: ☐Completed Application Open to all Optometrists? ☐Yes ☐No Maintain Record Agreement? ☐Yes ☐No ☐Correct Application Fee ☐Detailed Course Summary ☐Detailed Course Outline ☐PowerPoint and/or other Presentation Materials ☐Advertising (optional) ☐CV for EACH Course Instructor ☐License Verification for Each Course Instructor Disciplinary History? ☐Yes ☐No 1 Course Title: 10 Diagnoses That Are Not Dry Eye, but could save your patient’s eye and vision & Wetlab Course Presentation date: 3/29/17 Speaker: Jennifer Lee Wu, MD Target Audience: This lecture is intended for optometrist seeking continuing education Course Description: This lecture seeks to provide optometrists with information regarding Dry Eye Management and Ocular Surface Disease. Discussion includes patient symptoms and treatments to include carcinoma, inflammation, conjunctivitis and more. Lecture will include case study discussion, common misdiagnosis and management. Wetlab practical will provide amino disk demonstration and hands- on applications using pig eyes. Amino disks provided by various vendors. CE Credit: 2 CE Units 3 2/8/2017 1 10 DIAGNOSES THAT ARE NOT DRY EYE BUT COULD SAVE YOUR PATIENT’S LIFE AND VISION 2 COMMON SYMPTOMS OF DRY EYE DISEASE • Sensitivity to light • Redness • Fluctuating vision • Dull aching pain/sharp stabbing pain • Sandy/foreign body sensation • Excessive tearing • Headache • Itchy eyes • Morning crustiness • 3 WHAT DO YOU THINK WHEN YOU SEE A DRY EYE PATIENT ON YOUR SCHEDULE? 4 “WHY ARE YOU TELLING ME MY EYES ARE DRY WHEN I’M OVERFLOWING WITH TEARS?” “DOC I TRIED THE ARTIFICIAL TEAR BUT MY EYES ARE STILL RED AND I WAKE UP WITH MY EYES CRUSTED SHOT . -

Ocular Manifestations of Inherited Diseases Maya Eibschitz-Tsimhoni
10 Ocular Manifestations of Inherited Diseases Maya Eibschitz-Tsimhoni ecognizing an ocular abnormality may be the first step in Ridentifying an inherited condition or syndrome. Identifying an inherited condition may corroborate a presumptive diagno- sis, guide subsequent management, provide valuable prognostic information for the patient, and determine if genetic counseling is needed. Syndromes with prominent ocular findings are listed in Table 10-1, along with their alternative names. By no means is this a complete listing. Two-hundred and thirty-five of approxi- mately 1900 syndromes associated with ocular or periocular manifestations (both inherited and noninherited) identified in the medical literature were chosen for this chapter. These syn- dromes were selected on the basis of their frequency, the char- acteristic or unique systemic or ocular findings present, as well as their recognition within the medical literature. The boldfaced terms are discussed further in Table 10-2. Table 10-2 provides a brief overview of the common ocular and systemic findings for these syndromes. The table is organ- ized alphabetically; the boldface name of a syndrome is followed by a common alternative name when appropriate. Next, the Online Mendelian Inheritance in Man (OMIM™) index num- ber is listed. By accessing the OMIM™ website maintained by the National Center for Biotechnology Information at http://www.ncbi.nlm.nih.gov, the reader can supplement the material in the chapter with the latest research available on that syndrome. A MIM number without a prefix means that the mode of inheritance has not been proven. The prefix (*) in front of a MIM number means that the phenotype determined by the gene at a given locus is separate from those represented by other 526 chapter 10: ocular manifestations of inherited diseases 527 asterisked entries and that the mode of inheritance of the phe- notype has been proven. -

Eyelid Disease and Surgery Mark Bobofchak, DVM, DACVO Eye Care for Animals Cleveland, OH
Eyelid Disease and Surgery Mark Bobofchak, DVM, DACVO Eye Care for Animals Cleveland, OH The eyelids of domestic animals have many important functions. They are important in protecting the globe, contributing to and spreading the tear film, and preventing the buildup of ocular pathogens and environmental debris. This article will review the basics of eyelid anatomy and function as well as discuss common problems associated with these structures The eyelids near the margin are thought to be composed of four primary layers: the conjunctiva, the tarsus and stroma, the orbicularis oculi muscle, and the dermis/epidermis. The margin is a mucocutaneous junction that connects the conjunctiva on the bulbar surface with the haired skin of the external surface. The primary muscle involved in eyelid closure is the circumferential orbicularis oculi muscle, innervated by the facial nerve (CN VII). The primary muscle involved in opening of the eyelid is the levator palpebrae superioris innervated by the oculomotor nerve (CN III). The tarsus (stroma) is comprised of connective tissue, levator muscles, and the tarsal or meibomian glands. These glands secrete the lipid component of the tear film and the openings for the secretory ducts are visible along the eyelid margin. The palpebral conjunctiva is the smooth, inner lining of the eyelid. It is continuous with the bulbar conjunctiva and contains lymphoid follicles, goblet cells (secrete the mucous portion of the tear film) and lymphatics. Congenital abnormalities of the eyelids are occasionally seen in both dogs and cats. Physiologic ankyloblepharon is typically present until 10-14 days after birth. Premature opening of the palpebral fissure can result in severe ulceration since the lacrimal tissue is not fully developed.