Ocular Manifestations of Ectodermal Dysplasia Daphna Landau Prat1,2 , William R
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Cone Interaction with Progressive Macular Dysfunction
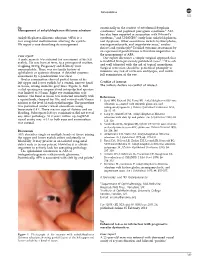
Correspondence 823 Sir, occasionally in the context of ectodermal dysplasia Management of ankyloblepharon filiforme adnatum syndromes3 and popliteal pterygium syndrome.4 AFA has also been reported in association with Edward’s Ankyloblepharon filiforme adnatum (AFA) is a syndrome,5 and CHANDS6 (curly hair, ankyloblepharon, rare congenital malformation affecting the eyelids. nail dysplasia). Other associations include hydrocephalus, We report a case describing its management. meningomyelocoele, and imperforate anus,7 cardiac defects and syndactyly.4 Detailed systemic assessment by an experienced paediatrician is therefore imperative in Case report the management of AFA. Our report illustrates a simple surgical approach that A male neonate was referred for assessment of his left is modified from previously published cases.1,2,4 It is safe eyelids. He was born at term, to a primigravid mother, and well tolerated with the aid of topical anaesthesia. weighing 3150 g. Pregnancy and delivery were Surgical correction should be performed promptly to unremarkable. There was no family history of minimise any risk of occlusion amblyopia, and enable ophthalmic or systemic disease. A detailed systemic full examination of the eye. assessment by a paediatrician was clear. Ocular examination showed partial fusion of his left upper and lower eyelids by a central, narrow band Conflict of interest of tissue, arising from the grey lines (Figure 1). Full The authors declare no conflict of interest. eyelid opening was impaired and interpalpebral aperture was limited to 3.5 mm. Right eye examination was normal. The band of tissue was retracted anteriorly with References a squint hook, clamped for 10 s, and excised with Vannas 1 Scott MH, Richard JM, Farris BK. -

Novel Anterior Segment Phenotypes Resulting from Forkhead Gene Alterations: Evidence for Cross-Species Conservation of Function
Novel Anterior Segment Phenotypes Resulting from Forkhead Gene Alterations: Evidence for Cross-Species Conservation of Function Ordan J. Lehmann,1 Stephen Tuft,2 Glen Brice,3 Richard Smith,4 Åsa Blixt,5 Rachel Bell,3 Bengt Johansson,6 Tim Jordan,1 Roger A. Hitchings,2 Peng T. Khaw,2 Simon W. M. John,4 Peter Carlsson,5 and Shomi S. Bhattacharya1 PURPOSE. Mutations in murine and human versions of an ances- cause it may affect the clinical management of certain trally related gene usually result in similar phenotypes. How- glaucoma subtypes and lead to excessive treatment. The ever, interspecies differences exist, and in the case of two FOXC1 and Foxe3 data, taken together with the novel ocular forkhead transcription factor genes (FOXC1 and FOXC2), phenotypes of FOXC2 mutations, highlight the remarkable these differences include corneal or anterior segment pheno- cross-species conservation of function among forkhead genes. types, respectively. This study was undertaken to determine (Invest Ophthalmol Vis Sci. 2003;44:2627–2633) DOI:10.1167/ whether such discrepancies provide an opportunity for iden- iovs.02-0609 tifying novel human–murine ocular phenotypes. METHODS. Four pedigrees with early-onset glaucoma pheno- types secondary to segmental chromosomal duplications or ecognition that mutations in orthologous genes frequently deletions encompassing FOXC1 and 18 individuals from 9 Rcause similar phenotypes has allowed the field of compar- FOXC2 mutation pedigrees underwent detailed ocular pheno- ative genetics to contribute to the understanding of human typing. Subsequently, mice with mutations in Foxc1 or a re- disease. As the human, murine, and Drosophila PAX6 mutants lated forkhead gene, Foxe3, were assessed for features of the (aniridia, Small eye, and eyeless) demonstrate, genotypic con- human phenotypes. -

Distichiasis (Distichia)
DISTICHIASIS (DISTICHIA) What is distichiasis? Distichiasis is a common condition that affects dogs, and less commonly, cats. The normal eyelid margin is devoid of hairs. There are multiple glands (called meibomian glands) along the eyelids which produce an oily secretion. Occasionally a hair can arise from or near these glands and project out of the eyelid. This hair is called a distichium, and the plural is distichia. These hairs may or may not come in contact with the cornea. This depends on whether they are thick/stiff or fine, and in which direction they are growing (they may be directed inwards). What problems do they cause? Some distichia do not cause any problems. These are usually fine hairs, or hairs which are directed away from the cornea. Problems arise when the hairs rub on the cornea. It causes discomfort, and the animal may have a watery eye discharge and have excessive blinking. The hair can rub on the cornea and abrade it, causing a corneal ulcer. These corneal ulcers are very difficult to treat, as the hair continues to rub in the same area, preventing healing. Distichia grow in young dogs, and if they are going to cause a problem, they usually do so ion dogs of less than two years of age. However, occasionally they can become problematic in later life, particularly if the dog gets a condition called Dry Eye or keratoconjunctivitis sicca. In this condition, there is a deficiency in tears, making the eyes dry and more susceptible to the abrading effect of the distichia. How is distichiasis diagnosed? Distichia may be seen with the naked eye with very close inspection. -

Canine Red Eye Elizabeth Barfield Laminack, DVM; Kathern Myrna, DVM, MS; and Phillip Anthony Moore, DVM, Diplomate ACVO
PEER REVIEWED Clinical Approach to the CANINE RED EYE Elizabeth Barfield Laminack, DVM; Kathern Myrna, DVM, MS; and Phillip Anthony Moore, DVM, Diplomate ACVO he acute red eye is a common clinical challenge for tion of the deep episcleral vessels, and is characterized general practitioners. Redness is the hallmark of by straight and immobile episcleral vessels, which run Tocular inflammation; it is a nonspecific sign related 90° to the limbus. Episcleral injection is an external to a number of underlying diseases and degree of redness sign of intraocular disease, such as anterior uveitis and may not reflect the severity of the ocular problem. glaucoma (Figures 3 and 4). Occasionally, episcleral Proper evaluation of the red eye depends on effective injection may occur in diseases of the sclera, such as and efficient diagnosis of the underlying ocular disease in episcleritis or scleritis.1 order to save the eye’s vision and the eye itself.1,2 • Corneal Neovascularization » Superficial: Long, branching corneal vessels; may be SOURCE OF REDNESS seen with superficial ulcerative (Figure 5) or nonul- The conjunctiva has small, fine, tortuous and movable vessels cerative keratitis (Figure 6) that help distinguish conjunctival inflammation from deeper » Focal deep: Straight, nonbranching corneal vessels; inflammation (see Ocular Redness algorithm, page 16). indicates a deep corneal keratitis • Conjunctival hyperemia presents with redness and » 360° deep: Corneal vessels in a 360° pattern around congestion of the conjunctival blood vessels, making the limbus; should arouse concern that glaucoma or them appear more prominent, and is associated with uveitis (Figure 4) is present1,2 extraocular disease, such as conjunctivitis (Figure 1). -

Congenital Ocular Anomalies in Newborns: a Practical Atlas
www.jpnim.com Open Access eISSN: 2281-0692 Journal of Pediatric and Neonatal Individualized Medicine 2020;9(2):e090207 doi: 10.7363/090207 Received: 2019 Jul 19; revised: 2019 Jul 23; accepted: 2019 Jul 24; published online: 2020 Sept 04 Mini Atlas Congenital ocular anomalies in newborns: a practical atlas Federico Mecarini1, Vassilios Fanos1,2, Giangiorgio Crisponi1 1Neonatal Intensive Care Unit, Azienda Ospedaliero-Universitaria Cagliari, University of Cagliari, Cagliari, Italy 2Department of Surgery, University of Cagliari, Cagliari, Italy Abstract All newborns should be examined for ocular structural abnormalities, an essential part of the newborn assessment. Early detection of congenital ocular disorders is important to begin appropriate medical or surgical therapy and to prevent visual problems and blindness, which could deeply affect a child’s life. The present review aims to describe the main congenital ocular anomalies in newborns and provide images in order to help the physician in current clinical practice. Keywords Congenital ocular anomalies, newborn, anophthalmia, microphthalmia, aniridia, iris coloboma, glaucoma, blepharoptosis, epibulbar dermoids, eyelid haemangioma, hypertelorism, hypotelorism, ankyloblepharon filiforme adnatum, dacryocystitis, dacryostenosis, blepharophimosis, chemosis, blue sclera, corneal opacity. Corresponding author Federico Mecarini, MD, Neonatal Intensive Care Unit, Azienda Ospedaliero-Universitaria Cagliari, University of Cagliari, Cagliari, Italy; tel.: (+39) 3298343193; e-mail: [email protected]. -

Lid Splitting and Posterior Lamellar Cryotherapy for Congenital Distichiasis and Trichiasis in Dog
Scientific Works. Series C. Veterinary Medicine. Vol. LXI (1) ISSN 2065-1295; ISSN 2343-9394 (CD-ROM); ISSN 2067-3663 (Online); ISSN-L 2065-1295 LID SPLITTING AND POSTERIOR LAMELLAR CRYOTHERAPY FOR CONGENITAL DISTICHIASIS AND TRICHIASIS IN DOG 1Andra ENACHE, 2Pip BOYDELL, 1Iuliana IONAŞCU, 1Alexandru ŞONEA 1University of Agronomical Sciences and Veterinary Medicine, Faculty of Veterinary Medicine, Bucharest, Romania, [email protected] 2 Animal Medical Centre Referral Services, Manchester, United Kingdom Corresponding author email: [email protected] Abstract Various surgical techniques have been proposed for treating distichiasis in dogs. A technique involving eyelid splitting and double freeze-thaw cryotherapy with anterior lamellar recession was evaluated. A 3 year old, female, Staffordshire bull terrier was referred for bilateral distichiasis. There were bilateral multiple distichiasis of the upper lids, more severe on the right lid with double row of cilia and two cilia on the lower lid. Under general anaesthesia, the eyelid margin was split at the gray line and a cryoprobe was used to freeze the posterior lamella. A double freeze-thaw technique was applied in both eyes. Anterior lamellar recession was performed to prevent postoperative entropion with trichiasis. The anterior and posterior lamellas were sutured with a 6/0 Vicryl suture. Bilateral upper eyelid edema was noted postoperatively. A month follow-up revealed increased bilateral granulation and depigmentation and the recurrence of one follicle on the right upper lid. Five months postoperatively there was no recurrence in the left eye but three cilia were detected in the right upper lid. The follicles have regrown due to incomplete destruction of the roots. -

Ocular Emergencies for the Primary Care Optometrist
Ocular Emergencies Ocular Emergencies for the Disclosure Statement Primary Care Optometrist . Honorarium, Speaker, Consultant, Research Grant: Aerie, Alcon, Allergan, B+L, Carl Zeiss, Glaukos, Heidelberg, Novartis, Topcon, Michael Chaglasian, OD, FAAO Associate Professor Illinois Eye Institute Illinois College of Optometry [email protected] What is a “True” Emergency? “True” Emergency . Pain (vs. discomfort) . History is key to differentiating emergency versus urgency . Current or potential for: Phone or in person Vision loss Proper triage is essential Structural damage After hours protocol Needs immediate (same day) attention Your office and your specialists Medico-legal implications History Emergency Exam Vision Recent ocular disease or One or both eyes? surgery . Acuity . External examination Visual field Other diseases . Visual fields . SLE Sudden or gradual cardiac, vascular, or . Pupils Blurred or lost? autoimmune . IOP Diplopia? viruses . Ocular Motility . Fundus exam Mono or Bino Medications or recent Pain changes to medications Redness Nausea/vomiting Onset Trauma Contact lenses M. Chaglasian, OD 1 Ocular Emergencies Emergency Kit “True” Emergency . Chemical Burns . Eye shield . pH paper Alkaline . Pressure patch . Bandage CL’s . Sterile eye wash . Diamox . Central Retinal Artery Occlusion . Alger brush . Topical drops . Forceps Antibiotics NSAID’s . Golf spud . Both have extremely high risk of severe and permanent Steroids vision loss which can be prevented via immediate Cycloplegics intervention and treatment Chemical Trauma Chemical Burns . Copious irrigation anesthetic . Acid exposure speculum Only penetrate through epithelium sterile saline v tap water car battery, vinegar, and some refrigerants . Contacts can be removed after irrigation . Sweep fornices – repeatedly . Alkaline exposure Penetrates tissues more easily and . Examination after irrigation and neutralization of pH have a prolonged effect . -

International Classification of Diseases
INTERNATIONAL CLASSIFICATION OF DISEASES MANUAL OF THE INTERNATIONAL STATISTICAL CLASSIFICATION OF DISEASES, INJURIES, AND CAUSES OF DEATH Based on the Recommendations of the Eighth Revision Conference, 1965, and Adopted by the Nineteenth World Health Assembly Volume 2 ALPHABETICAL INDEX WORLD HEALTH ORGANIZATION GENEVA 1969 Volume 1 Introduction List of Three-digit Categories Tabular List of Inclusions and Four-digit Sub- categories Medical Certification and Rules for Classification Special Lists for Tabulation Definitions and Recommendations Regulations Volume 2 Alphabetical Index PRINTED IN ENGLAND CONTENTS Introduction Page General arrangement of the Index ....................................... VIII Main sections ............................................................... VIII Structure ..................................................................... IX Code numbzrs .............................................................. x Primary and secondary conditions. ................................... x Multiple diagnoses. ........................................................ XI Spelling....................................................................... XI Order of listing ............................................................. Conventions used in the Index ........................................... XII Parentheses. ................................................................. XII Cross-referexes ........................................................... XI1 Abbreviation NEC. ...................................................... -

The 25Th Annual Waltham/OSU Symposium Small Animal Ophthalmology October 27—28, 2001
The 25th Annual Waltham/OSU Symposium Small Animal Ophthalmology October 27—28, 2001 Conditions of the Eyelids and Ocular Adnexa in Dogs and Cats ________________________________________ David T. Ramsey, DVM, Diplomate ACVO Associate Professor, Comparative Ophthalmology, Department of Small Animal Clinical Sciences D-208 Veterinary Medical Center Michigan State University East Lansing, MI 48824-1314 ________________________________________ The eyelids and ocular adnexa comprise the primary surface defense for the globe, specifically the cornea. Physical or functional abnormalities of the lids or adnexal ocular structures may result in abnormalities of the cornea and subsequently vision. This commentary will discuss diagnosis and treatment of the more common abnormalities affecting the lids and ocular adnexa in dogs and cats. Anatomy The eyelids represent a composite structure composed of skin and cutaneous appendages, including hair follicles and glandular structures. Dogs have eyelashes only on the upper lid and cats lack upper and lower eyelashes. The palpebral conjunctiva is a highly vascular mucous membrane that lines the inner aspect of the lids. Interposed between the surface skin and palpebral conjunctiva is skeletal muscle and fibrous tissue. Eyelids are highly vascularized, therefore they are fairly resistant to microbial infection and have the property of healing rapidly after suffering trauma, but may enlarge considerably from transvascular exudation of fluid into lid tissue when irritated. Since skin comprises a substantial portion of the eyelid, a plethora of dermatologic conditions may affect the eyelids.1 The adnexa consists of all segments of conjunctiva (palpebral, nictitans, bulbar and fornix), and the nictitating membrane. The conjunctiva is the most exposed mucous membrane of the body. -

The EEC Syndrome and Its Ocular Manifestations
Br J Ophthalmol: first published as 10.1136/bjo.73.4.261 on 1 April 1989. Downloaded from British Journal ofOphthalmology, 1989, 73, 261-264 The EEC syndrome and its ocular manifestations ALAN A McNAB,* MICHAEL J POTTS, AND RICHARD A N WELHAM From the Lacrimal Clinic, Moorfields Eye Hospital, London, EC] V2PD SUMMARY The EEC syndrome (ectrodactyly or lobster-claw deformity, ectodermal dysplasia, and cleft lip and palate) is a rare disorder with autosomal dominant inheritance, variable expression, and in some families lack of penetrance. We present the findings in five cases with emphasis on the ocular findings. Lacrimal surgery was performed on three patients with good results in each case. We also report the occurrence of spontaneous corneal perforation in two cases, a complication not previously recognised. The ophthalmic care of these patients must be pursued long-term, as progressive visual impairment may be the most disabling feature of the syndrome. The EEC syndrome, and acronym coined by Rudiger provide a more functional hand. Both feet had et al in 1970,' was first recognised as a distinct clinical syndactyly. Her hair was coarse and dry and her skin entity by Cockayne.2 He drew attention to the subject to eczema. She had a bilateral conductive dacryocystitis which commonly afflicts these patients, hearing loss and wore hearing aids. There was and found that it was associated with 'atresia of the bilateral malar hypoplasia and small malformed lacrimal ducts.' Since that description in 1936, several teeth. other reports have appeared in the ophthalmic We first saw her at the age of 11 years. -

Obstructed Tear Duct Causes Epiphora and Precocious Eyelid Opening Due
bioRxiv preprint doi: https://doi.org/10.1101/2020.04.17.046383; this version posted April 17, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Obstructed tear duct causes epiphora and precocious eyelid opening due to disruption of Prickle 1-mediated Wnt/PCP signaling Dianlei Guo1*, Jiali Ru1*, Jiaying Fan2*, Rong Ju1, Kangxin Jin1, Hong Ouyang1, Lai Wei1, Yizhi Liu1, Chunqiao Liu1$ 1, State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, Sun Yat-sen University, Guangzhou 510060, China 2, Guangzhou Woman & Children’s Medical Center *Equal contribution $Correspondence should be addressed to Dr. Chunqiao Liu: Email: [email protected] bioRxiv preprint doi: https://doi.org/10.1101/2020.04.17.046383; this version posted April 17, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Abstract The tear drainage apparatus evolved in terrestrial animals serving as conduits for tear flow. Obstruction of tear drainage causes a range of ocular surface disorders. Hitherto, genetics of tear duct development and obstruction has been scarcely explored. Here we report that a severe Prickle 1 hypomorph mouse line exhibited epiphora. This phenotype was due to blockage of the tear drainage by the incompletely formed nasolacrimal duct (NLD) and lacrimal canaliculi (CL). -

10867 Ocular Vol 9 #1.Indd
Volume 9, Issue 1 October 2010 ® A Quarterly Publication for the Veterinary Community from Eye Care for Animals When Hairs Meet the Eye become shortened the facial skin has sequestra. In some dogs we see corneal not correspondingly been reduced - this vascularization and medial corneal results in the formation of large nasal pigmentation as a result of the chronic folds of haired skin. Hair on the nasal cilia irritation. This pathology is often folds may impinge on the eye and cause compounded by the abnormally large problems in some dogs. As the facial palpebral fissure (macroblepharon), the skin is pushed towards the eye there is shallow orbit which limits the extent to a tendency for the lower eyelid to roll which the eye can be retracted and the against the eye (entropion). This is lids closed over the cornea, the extreme usually compounded by tight attachment rounded (rather than elliptical) shape of the medial canthus (the location where of the upper and lower eyelids around the upper and lower eyelids join on the the medial canthus which may reduce nasal side of the face) to the underlying the effective medial corneal coverage bone via the medial palpebral ligament. during blinking, and in some dogs Nicholas J. Millichamp, The net result is a rolling of the medial reduced tear secretion from the lacrimal BVetMed, PhD, DVOphthal, DECVO, part of the lower (and occasionally glands (keratoconjunctivitis sicca). All MRCVS, DACVO upper) eyelid such that normal eyelid of these factors (and probably some that Eye Care for Animals cilia contact the conjunctiva or cornea.