Obstructed Tear Duct Causes Epiphora and Precocious Eyelid Opening Due
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Low Level Light Therapy for the Treatment of Recalcitrant Chalazia: a Sample Case Summary
Clinical Ophthalmology Dovepress open access to scientific and medical research Open Access Full Text Article ORIGINAL RESEARCH Low level light therapy for the treatment of recalcitrant chalazia: a sample case summary This article was published in the following Dove Press journal: Clinical Ophthalmology Karl Stonecipher1 Purpose: To evaluate the effects of low-level light therapy (LLLT) on the resolution of Richard Potvin 2 recalcitrant chalazia. Patients and Methods: This was a single-site retrospective chart review of patients with 1Physicians Protocol, Greensboro, NC, USA; 2Science in Vision, Akron, NY, USA chalazia, all of whom were unresponsive to previous pharmaceutical therapy or surgical intervention, who received a 15 min LLLT treatment in conjunction with a standard phar- maceutical regimen. A second treatment was applied 24 hrs to as late as 2 months if there was no evidence of progression of resolution in appearance. Results: A total of 26 eyes of 22 patients with relevant history and treatment were reviewed, all with a history of prior pharmaceutical treatment for their chalazia. After a single 15 min LLLT treatment, followed by a standard pharmaceutical regimen, 46% of eyes (12/26) showed resolution of their chalazia. Resolution was noted from 3 days to one-month post- treatment. With a second treatment, the chalazia resolved in 92% of eyes (24/26). Only two For personal use only. eyes of the 26 (8%) required incision and curettage after LLLT treatment. Conclusion: The use of LLLT for the treatment of recalcitrant chalazia appears to be beneficial in patients who have failed topical and/or systemic therapy, significantly reducing the likelihood of requiring surgical intervention. -
Cone Interaction with Progressive Macular Dysfunction
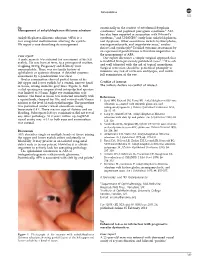
Correspondence 823 Sir, occasionally in the context of ectodermal dysplasia Management of ankyloblepharon filiforme adnatum syndromes3 and popliteal pterygium syndrome.4 AFA has also been reported in association with Edward’s Ankyloblepharon filiforme adnatum (AFA) is a syndrome,5 and CHANDS6 (curly hair, ankyloblepharon, rare congenital malformation affecting the eyelids. nail dysplasia). Other associations include hydrocephalus, We report a case describing its management. meningomyelocoele, and imperforate anus,7 cardiac defects and syndactyly.4 Detailed systemic assessment by an experienced paediatrician is therefore imperative in Case report the management of AFA. Our report illustrates a simple surgical approach that A male neonate was referred for assessment of his left is modified from previously published cases.1,2,4 It is safe eyelids. He was born at term, to a primigravid mother, and well tolerated with the aid of topical anaesthesia. weighing 3150 g. Pregnancy and delivery were Surgical correction should be performed promptly to unremarkable. There was no family history of minimise any risk of occlusion amblyopia, and enable ophthalmic or systemic disease. A detailed systemic full examination of the eye. assessment by a paediatrician was clear. Ocular examination showed partial fusion of his left upper and lower eyelids by a central, narrow band Conflict of interest of tissue, arising from the grey lines (Figure 1). Full The authors declare no conflict of interest. eyelid opening was impaired and interpalpebral aperture was limited to 3.5 mm. Right eye examination was normal. The band of tissue was retracted anteriorly with References a squint hook, clamped for 10 s, and excised with Vannas 1 Scott MH, Richard JM, Farris BK. -

Differentiate Red Eye Disorders
Introduction DIFFERENTIATE RED EYE DISORDERS • Needs immediate treatment • Needs treatment within a few days • Does not require treatment Introduction SUBJECTIVE EYE COMPLAINTS • Decreased vision • Pain • Redness Characterize the complaint through history and exam. Introduction TYPES OF RED EYE DISORDERS • Mechanical trauma • Chemical trauma • Inflammation/infection Introduction ETIOLOGIES OF RED EYE 1. Chemical injury 2. Angle-closure glaucoma 3. Ocular foreign body 4. Corneal abrasion 5. Uveitis 6. Conjunctivitis 7. Ocular surface disease 8. Subconjunctival hemorrhage Evaluation RED EYE: POSSIBLE CAUSES • Trauma • Chemicals • Infection • Allergy • Systemic conditions Evaluation RED EYE: CAUSE AND EFFECT Symptom Cause Itching Allergy Burning Lid disorders, dry eye Foreign body sensation Foreign body, corneal abrasion Localized lid tenderness Hordeolum, chalazion Evaluation RED EYE: CAUSE AND EFFECT (Continued) Symptom Cause Deep, intense pain Corneal abrasions, scleritis, iritis, acute glaucoma, sinusitis, etc. Photophobia Corneal abrasions, iritis, acute glaucoma Halo vision Corneal edema (acute glaucoma, uveitis) Evaluation Equipment needed to evaluate red eye Evaluation Refer red eye with vision loss to ophthalmologist for evaluation Evaluation RED EYE DISORDERS: AN ANATOMIC APPROACH • Face • Adnexa – Orbital area – Lids – Ocular movements • Globe – Conjunctiva, sclera – Anterior chamber (using slit lamp if possible) – Intraocular pressure Disorders of the Ocular Adnexa Disorders of the Ocular Adnexa Hordeolum Disorders of the Ocular -

Keratoconjunctivitis Sicca (“Dry Eye”) Stephanie Shrader, DVM and John Robertson, VMD, Phd
Keratoconjunctivitis sicca (“Dry Eye”) Stephanie Shrader, DVM and John Robertson, VMD, PhD Introduction and Overview How Tears Are Produced Keratoconjunctivitis sicca (KCS) is a disease of the eyes, The tear film that covers the eyes is made up of three distinct characterized by inflammation of the cornea and conjunctiva. layers. The outermost layer is made up of oils, which are This condition occurs secondary to a deficiency in formation secreted by the Meibomian glands. This lipid layer provides of the tear film that normally protects the cornea (Best et al, protection against evaporation, binds the tear film to the 2014), which leads to dry, irritated eyes. As a result, KCS is cornea, and prevents tears from simply pouring out over the commonly known as “dry eye” or in veterinary terminology, lower eyelid onto the face. The middle layer of the tear film is xerophthalmia. This disease occurs often in West Highland the aqueous layer, which is produced by the lacrimal glands. As White Terriers, but is also common in many other breeds, its name would suggest, the aqueous layer consists primarily including Lhasa Apso, English Bulldog, American Cocker of water, along with important proteins and enzymes that help Spaniel, English Springer Spaniel, Pekingese, Pug, Chinese Shar remove bacteria and cellular waste material, and lubricate the Pei, Yorkshire Terrier, Shih Tzu, Miniature Schnauzer, German surface of the cornea. The innermost layer of the tear film is the Shepherd, Doberman Pinscher, and Boston Terrier. While the mucin layer, which is is produced by tiny secretory cells in the reported incidence of KCS across all dog breeds ranges from conjunctiva known as goblet cells. -

Epiphora During the First Year of Life
Eye (1991) 5, 596--600 Epiphora During the First Year of Life C. J. MACEWEN and J. D. H. YOUNG Dundee Summary A cohort of 4,792 infants was observed in order to determine the incidence and natu ral history of epiphora during the first year of life. Evidence of defective lacrimal drainage was present in 964 (20%) at some time during the year. 9S�/o became symp tomatic during the first month of life. Spontaneous remission occurred throughout the year and 96% had resolved before the age of one. This study provides no evidence to support probing before the age of one year. Infants with epiphora are a common problem haps the most reliable estimate of the inci in clinical ophthalmology. It is generally dence is 6%. This comes from a follow-up accepted that the condition is the result of a study of 200 consecutive, unselected newborn congenital abnormality of the lacrimal drain infants.7 age system, in the form of a membranous Information on the rate of spontaneous obstruction at the lower end of the naso-lac remission is also limited. The studies that are rimal duct (NLD). I In addition it is recognised available were based on small clinic popula that there is a high rate of spontaneous resol tions, referred for treatment of their epiphora ution.2•3 However, despite it's frequency, rela and probing was usually undertaken in a tively little is known about the incidence or number of cases before the end of the year.2,3 natural history of epiphora in young children. -

Cytokine Profiles of Tear Fluid from Patients with Pediatric Lacrimal
Immunology and Microbiology Cytokine Profiles of Tear Fluid From Patients With Pediatric Lacrimal Duct Obstruction Nozomi Matsumura,1 Satoshi Goto,2 Eiichi Uchio,3 Kyoko Nakajima,4 Takeshi Fujita,1 and Kazuaki Kadonosono5 1Department of Ophthalmology, Kanagawa Children’s Medical Center, Yokohama, Japan 2Department of Ophthalmology, The Jikei University School of Medicine, Tokyo, Japan 3Department of Ophthalmology, Faculty of Medicine, Fukuoka University, Fukuoka, Japan 4Department of Joint Laboratory for Frontier Medical Science, Faculty of Medicine, Fukuoka University, Fukuoka, Japan 5Department of Ophthalmology and Micro-technology, Yokohama City University, Yokohama, Japan Correspondence: Nozomi Matsu- PURPOSE. This study evaluated the cytokine levels in unilateral tear samples from both sides in mura, Department of Ophthalmolo- patients with pediatric lacrimal duct obstruction. gy, Kanagawa Children’s Medical Center, 2-138-4 Mutsukawa, Minami- METHODS. Fifteen cases of unilateral lacrimal duct obstruction (mean, 26.9 6 28.7 months old) ku, Yokohama 232-8555, Japan; were enrolled in this study. Tear samples were collected separately from the obstructed side [email protected]. and the intact side in each case before surgery, which was performed under general Submitted: September 8, 2016 anesthesia or sedation. The levels of IL-2, IL-4, IL-6, IL-10, TNF, IFN-c, and IL-17A then were Accepted: December 2, 2016 measured in each tear sample. A receiver operating characteristic (ROC) curve was constructed for the IL-6 levels in the tears. We also measured the postoperative tear fluid Citation: Matsumura N, Goto S, Uchio levels of IL-6 in those cases from which tear fluid samples could be collected after the surgery. -

Scleral Faqs
www.scleralcenter.com SCLERAL FAQS CONTACT US: Office: 501 E. Palm Valley Blvd. WHY ARE SCLERAL LENSES SO Round Rock, TX 78664 COMFORTABLE? Website: www.scleralcenter.com Scleral lenses vault over the cornea and rest on Phone: 512.248.2424 the white portion of the eye (sclera), which is less sensitive. They fit under the eyelid, resulting in comfort & stability. JAVIER R. ZAMORA, OD Dr. Zamora is the co-founder of Advanced Eye Care & Surgery and has been fitting specialty contact lenses since 1998. He is a graduate of the University of Texas at Austin and the University of Houston College of Optometry. FIRM LENSES LARGER LENSES Sharp Vision Comfort & Stability DEBBIE A. ZAMORA, OD Dr. Zamora is the co- founder of Advanced Eye WHY DO SCLERAL LENSES OFFER Care & Surgery and has SUPERIOR VISION? been fitting specialty contact lenses since They are manufactured in Gas Permeable (GP) 2001. She is a graduate HOW material which provides a smooth optical surface of Tulane University and the University of Houston and excellent vision even if your cornea has an College of Optometry. SCLERAL LENSES irregular shape. Scleral lenses treat astigmatism and are available with bifocal options. CAN HELP YOU DEBRA A. WARE, OD WHAT IS THE ‘LIQUID BANDAGE’ Dr. Ware has been fitting EFFECT? specialty contact lenses since 1998. She is Scleral lenses provide extra moisture for healthy a graduate of Baylor eyes, as well as for patients with severe dry eyes. University and the The space between your eye and the back of the University of Houston scleral lens acts as a fluid reservoir that provides College of Optometry. -

Dry Eye in Patient with Clinical History of Chronic Blepharitis and Chalaziosis Edited by Dr
year 10 num b e r 2 4 e y e d o c t o r m a r ch- a p r i l 2018 CLINICAL CASES OF LUCIO BURATTO Dry eye in patient with clinical history of chronic blepharitis and chalaziosis edited by Dr. Maria Luisa Verbelli, Dr.Alessia Bottoni Observation and 1 anamnesis Arrives at our observation at CIOS, Italian Center for Dry Eye at CAMO, a 56-year-old patient with blepharitis, redness, ocular burning and abundant mucous secretion present in both eyes. Furthermore, an enlarged lymph node is seen in the right laterocervical site. At ocular anamnesis the patient reports chronic blepharitis from the juvenile age, multiple chalazion in both eyes, an operation for right Fig. 1 Handpiece for the application of the pulsed light of the Eye-Light instrument upper eyelid chalaziosis in 2006 (4 upper eyelid chalazion , 3 in the lower); negative anamnesis for these pathologies in the family. The patient is shortsighted since adolescence, has not had any other eye operations and has no ocular allergies. The general anamnesis does not report major systemic diseases or medication intake. On objective examination of the anterior segment we find bilaterally: reduced lacrimal meniscus, posterior blepharitis, obstruction of all the Meibomian glands of the upper and lower eyelids, conjunctival hyperemia with dry spots, transparent cornea, transparent crystalline. The no contact tonometry is 15 mmHg in RE, 16 mmHg in LE. The OCT of the macula does not show changes in both eyes. The BUT is 4.9 seconds in RE, and 15.6 seconds in LE. -

Olivia Steinberg ICO Primary Care/Ocular Disease Resident American Academy of Optometry Residents Day Submission
Olivia Steinberg ICO Primary Care/Ocular Disease Resident American Academy of Optometry Residents Day Submission The use of oral doxycycline and vitamin C in the management of acute corneal hydrops: a case comparison Abstract- We compare two patients presenting to clinic with an uncommon complication of keratoconus, acute corneal hydrops. Management of the patients differs. One heals quickly, while the other has a delayed course to resolution. I. Case A a. Demographics: 40 yo AAM b. Case History i. CC: red eye, tearing, decreased VA x 1 day OS ii. POHx: (+) keratoconus OU iii. PMHx: depression, anxiety, asthma iv. Meds: Albuterol, Ziprasidone v. Scleral CL wearer for approximately 6 months OU vi. Denies any pain OS, denies previous occurrence OU, no complaints OD c. Pertinent Findings i. VA cc (CL’s)- 20/25 OD, 20/200 PH 20/60+2 OS ii. Slit Lamp 1. Inferior corneal thinning and Fleisher ring OD, central scarring OD, 2+ diffuse microcystic edema OS, Descemet’s break OS (photos and anterior segment OCT) 2. 2+ diffuse injection OS 3. D&Q A/C OU iii. Intraocular Pressures: deferred OD due to CL, 9mmHg OS (tonopen) iv. Fundus Exam- unremarkable OU II. Case B a. Demographics: 39 yo AAM b. Case History i. CC: painful, red eye, tearing, decreased VA x 1 day OS ii. POHx: unremarkable iii. PMHx: hypertension iv. Meds: unknown HTN medication v. Wears Soflens toric CL’s OU; reports previous doctor had difficulty achieving proper fit OU; denies diagnosis of keratoconus OU vi. Denies any injury OS, denies previous occurrence OU, no complaints OD c. -

Etiology and Management of Epiphora in the Adult Patient: a Retrospective Study in an Interdisciplinary Epiphora Clinic
Research Article Journal of Clinical Ophthalmology and Eye Disorders Published: 26 Feb, 2021 Etiology and Management of Epiphora in the Adult Patient: A Retrospective Study in an Interdisciplinary Epiphora Clinic Vincent Q1*, Roxane F1, Aurelie L1 and Nicolas M2 1Department of Ophthalmology, UCLouvain, UCLouvain Catholic University of Louvain, Belgium 2Department of Otorhinolaryngology, UCLouvain, UCLouvain Catholic University of Louvain, Belgium Abstract Background: Epiphora in adults is a frequent ophthalmological condition with multiple etiologies, and requires a multidisciplinary approach for management, diagnosis and treatment, combining ophthalmologists, ENTs, nuclear medicine specialists, radiologists. Few centers possess a truly multi-specialty method for analyzing and treating epiphora in adults. Materials and Methods: We have conducted a retrospective study on 57 patients with a follow-up of 12 months, to examine the different etiologies and treatments in a multidisciplinary epiphora clinic in a tertiary care setting. Patients were systematically examined by an ophthalmologist and an ENT specialist, in addition to a full epiphora clinical workup. If needed, they were then referred for additional examinations in radiology (dacryo cone beam scanner) or scintigraphy. Results: Obstruction at any stage of the lacrimal drainage system was the most common cause of epiphora (48%), followed by ocular surface disease (28%), then eyelid malposition or laxity (26%), and finally functional causes. Regarding treatments, 10.5% (n=6) of patients underwent 3-snip punctoplasty, 8% (n=5) underwent canalicular repermeabilization through sharp catheterization, 21% (n=12) underwent DCR, 42% (n=24) were prescribed lid hygiene or ocular lubrication, 21% OPEN ACCESS (n=12) underwent eyelid surgery through canthopexy, 1% (n=1) had a combined treatment and 19% (n=11) had no treatment. -

Scleral Lenses and Eye Health
Scleral Lenses and Eye Health Anatomy and Function of the Human Eye How Scleral Lenses Interact with the Ocular Surface Just as the skin protects the human body, the ocular surface protects the human Scleral lenses are large-diameter lenses designed to vault the cornea and rest on the conjunctival tissue sitting on eye. The ocular surface is made up of the cornea, the conjunctiva, the tear film, top of the sclera. The space between the back surface of the lens and the cornea acts as a fluid reservoir. Scleral and the glands that produce tears, oils, and mucus in the tear film. lenses can range in size from 13mm to 19mm, although larger diameter lenses may be designed for patients with more severe eye conditions. Due to their size, scleral lenses consist SCLERA: The sclera is the white outer wall of the eye. It is SCLERAL LENS made of collagen fibers that are arranged for strength rather of at least two zones: than transmission of light. OPTIC ZONE The optic zone vaults over the cornea CORNEA: The cornea is the front center portion of the outer Cross section of FLUID RESERVOIR wall of the eye. It is made of collagen fibers that are arranged in the eye shows The haptic zone rests on the conjunctiva such a way so that the cornea is clear. The cornea bends light the cornea, overlying the sclera as it enters the eye so that the light is focused on the retina. conjunctiva, and sclera as CORNEA The cornea has a protective surface layer called the epithelium. -

Immune Defense at the Ocular Surface
Eye (2003) 17, 949–956 & 2003 Nature Publishing Group All rights reserved 0950-222X/03 $25.00 www.nature.com/eye Immune defense at EK Akpek and JD Gottsch CAMBRIDGE OPHTHALMOLOGICAL SYMPOSIUM the ocular surface Abstract vertebrates. Improved visual acuity would have increased the fitness of these animals and would The ocular surface is constantly exposed to a have outweighed the disadvantage of having wide array of microorganisms. The ability of local immune cells and blood vessels at a the outer ocular system to recognize pathogens distance where a time delay in addressing a as foreign and eliminate them is critical to central corneal infection could lead to blindness. retain corneal transparency, hence The first vertebrates were jawless fish that preservation of sight. Therefore, a were believed to have evolved some 470 million combination of mechanical, anatomical, and years ago.1 These creatures had frontal eyes and immunological defense mechanisms has inhabited the shorelines of ancient oceans. With evolved to protect the outer eye. These host better vision, these creatures were likely more defense mechanisms are classified as either a active and predatory. This advantage along with native, nonspecific defense or a specifically the later development of jaws enabled bony fish acquired immunological defense requiring to flourish and establish other habitats. One previous exposure to an antigen and the such habitat was shallow waters where lunged development of specific immunity. Sight- fish made the transition to land several hundred threatening immunopathology with thousand years later.2 To become established in autologous cell damage also can take place this terrestrial environment, the new vertebrates after these reactions.