Congenital Ocular Anomalies in Newborns: a Practical Atlas
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Congenital Cataract
3801 W. 15th St., Bldg. A, Ste. 110 Plano, TX 75075 Phone: (972) 758-0625 Fax: (972) 964-5725 Email: [email protected] Website: www.drstagerjr.com Congenital Cataract Your eye works a lot like a camera. Light rays focus through the lens on the retina, a layer of light-sensitive cells at the back of the eye. Similar to photographic film, the retina allows the image to be “seen” by the brain. Over time, the lens of our eye can become cloudy, preventing light rays from passing clearly through the lens. The loss of transparency may be so mild that vision is barely affected, or it can be so severe that no shapes or movements are seen—only light and dark. When the lens becomes cloudy enough to obstruct vision to any significant degree, it is called a cataract. Eyeglasses or contact lenses can usually correct slight refractive errors caused by early cataracts, but they cannot sharpen your vision if a severe cataract is present. The most common cause of cataract is aging. Occasionally, babies are born with cataracts or develop them very early in life. This condition is called congenital cataract. There are many causes of congenital cataract. Certain diseases can cause the condition, and sometimes it can be inherited. However, in most cases, there is no identifiable cause. Treatment for cataract in infants varies depending on the nature of each patient’s condition. Surgery is usually recommended very early in life, but many factors affect this decision, including the infant’s health and whether there is a cataract in one or both eyes. -
Cone Interaction with Progressive Macular Dysfunction
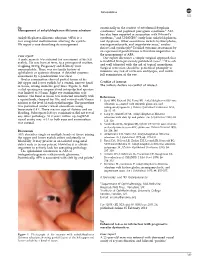
Correspondence 823 Sir, occasionally in the context of ectodermal dysplasia Management of ankyloblepharon filiforme adnatum syndromes3 and popliteal pterygium syndrome.4 AFA has also been reported in association with Edward’s Ankyloblepharon filiforme adnatum (AFA) is a syndrome,5 and CHANDS6 (curly hair, ankyloblepharon, rare congenital malformation affecting the eyelids. nail dysplasia). Other associations include hydrocephalus, We report a case describing its management. meningomyelocoele, and imperforate anus,7 cardiac defects and syndactyly.4 Detailed systemic assessment by an experienced paediatrician is therefore imperative in Case report the management of AFA. Our report illustrates a simple surgical approach that A male neonate was referred for assessment of his left is modified from previously published cases.1,2,4 It is safe eyelids. He was born at term, to a primigravid mother, and well tolerated with the aid of topical anaesthesia. weighing 3150 g. Pregnancy and delivery were Surgical correction should be performed promptly to unremarkable. There was no family history of minimise any risk of occlusion amblyopia, and enable ophthalmic or systemic disease. A detailed systemic full examination of the eye. assessment by a paediatrician was clear. Ocular examination showed partial fusion of his left upper and lower eyelids by a central, narrow band Conflict of interest of tissue, arising from the grey lines (Figure 1). Full The authors declare no conflict of interest. eyelid opening was impaired and interpalpebral aperture was limited to 3.5 mm. Right eye examination was normal. The band of tissue was retracted anteriorly with References a squint hook, clamped for 10 s, and excised with Vannas 1 Scott MH, Richard JM, Farris BK. -

National Study of Microphthalmia, Anophthalmia, and Coloboma (MAC
16 ORIGINAL ARTICLE J Med Genet: first published as 10.1136/jmg.39.1.16 on 1 January 2002. Downloaded from National study of microphthalmia, anophthalmia, and coloboma (MAC) in Scotland: investigation of genetic aetiology D Morrison, D FitzPatrick, I Hanson, K Williamson, V van Heyningen, B Fleck, I Jones, J Chalmers, H Campbell ............................................................................................................................. J Med Genet 2002;39:16–22 We report an epidemiological and genetic study attempting complete ascertainment of subjects with microphthalmia, anophthalmia, and coloboma (MAC) born in Scotland during a 16 year period beginning on 1 January 1981. A total of 198 cases were confirmed giving a minimum live birth preva- lence of 19 per 100 000. One hundred and twenty-two MAC cases (61.6%) from 115 different fami- See end of article for lies were clinically examined and detailed pregnancy, medical, and family histories obtained. A authors’ affiliations simple, rational, and apparently robust classification of the eye phenotype was developed based on ....................... the presence or absence of a defect in closure of the optic (choroidal) fissure. A total of 85/122 Correspondence to: (69.7%) of cases had optic fissure closure defects (OFCD), 12/122 (9.8%) had non-OFCD, and Dr D FitzPatrick, MRC 25/122 (20.5%) had defects that were unclassifiable owing to the severity of the corneal or anterior Human Genetics Unit, chamber abnormality. Segregation analysis assuming single and multiple incomplete ascertainment, Western General Hospital, respectively, returned a sib recurrence risk of 6% and 10% in the whole group and 8.1% and 13.3% Edinburgh EH4 2XU, UK; in the OFCD subgroup. -

Treatment of Congenital Ptosis
13 Review Article Page 1 of 13 Treatment of congenital ptosis Vladimir Kratky1,2^ 1Department of Ophthalmology, Queen’s University, Kingston, Canada; 21st Medical Faculty, Charles University, Prague, Czech Republic Correspondence to: Vladimir Kratky, BSc, MD, FRCSC, DABO. Associate Professor of Ophthalmology, Director of Ophthalmic Plastic and Orbital Surgery, Oculoplastics Fellowship Director, Queen’s University, Kingston, Canada; 1st Medical Faculty, Charles University, Prague, Czech Republic. Email: [email protected]. Abstract: Congenital ptosis is an abnormally low position of the upper eyelid, with respect to the visual axis in the primary gaze. It can be present at birth or manifest itself during the first year of life and can be bilateral or unilateral. Additionally, it may be an isolated finding or part of a constellation of signs of a specific syndrome or systemic associations. Depending on how much it interferes with the visual axis, it may be considered as a functional or a cosmetic condition. In childhood, functional ptosis can lead to deprivation amblyopia and astigmatism and needs to be treated. However, even mild ptosis with normal vision can lead to psychosocial problems and correction is also advised, albeit on a less urgent basis. Although, patching and glasses can be prescribed to treat the amblyopia, the mainstay of management is surgical. There are several types of surgical procedure available depending on the severity and etiology of the droopy eyelid. The first part of this paper will review the different categories of congenital ptosis, including more common associated syndromes. The latter part will briefly cover the different surgical approaches, with emphasis on how to choose the correct condition. -

Novel Anterior Segment Phenotypes Resulting from Forkhead Gene Alterations: Evidence for Cross-Species Conservation of Function
Novel Anterior Segment Phenotypes Resulting from Forkhead Gene Alterations: Evidence for Cross-Species Conservation of Function Ordan J. Lehmann,1 Stephen Tuft,2 Glen Brice,3 Richard Smith,4 Åsa Blixt,5 Rachel Bell,3 Bengt Johansson,6 Tim Jordan,1 Roger A. Hitchings,2 Peng T. Khaw,2 Simon W. M. John,4 Peter Carlsson,5 and Shomi S. Bhattacharya1 PURPOSE. Mutations in murine and human versions of an ances- cause it may affect the clinical management of certain trally related gene usually result in similar phenotypes. How- glaucoma subtypes and lead to excessive treatment. The ever, interspecies differences exist, and in the case of two FOXC1 and Foxe3 data, taken together with the novel ocular forkhead transcription factor genes (FOXC1 and FOXC2), phenotypes of FOXC2 mutations, highlight the remarkable these differences include corneal or anterior segment pheno- cross-species conservation of function among forkhead genes. types, respectively. This study was undertaken to determine (Invest Ophthalmol Vis Sci. 2003;44:2627–2633) DOI:10.1167/ whether such discrepancies provide an opportunity for iden- iovs.02-0609 tifying novel human–murine ocular phenotypes. METHODS. Four pedigrees with early-onset glaucoma pheno- types secondary to segmental chromosomal duplications or ecognition that mutations in orthologous genes frequently deletions encompassing FOXC1 and 18 individuals from 9 Rcause similar phenotypes has allowed the field of compar- FOXC2 mutation pedigrees underwent detailed ocular pheno- ative genetics to contribute to the understanding of human typing. Subsequently, mice with mutations in Foxc1 or a re- disease. As the human, murine, and Drosophila PAX6 mutants lated forkhead gene, Foxe3, were assessed for features of the (aniridia, Small eye, and eyeless) demonstrate, genotypic con- human phenotypes. -

2018 Etiologies by Frequencies
2018 Etiologies in Order of Frequency by Category Hereditary Syndromes and Disorders Count CHARGE Syndrome 958 Down syndrome (Trisomy 21 syndrome) 308 Usher I syndrome 252 Stickler syndrome 130 Dandy Walker syndrome 119 Cornelia de Lange 102 Goldenhar syndrome 98 Usher II syndrome 83 Wolf-Hirschhorn syndrome (Trisomy 4p) 68 Trisomy 13 (Trisomy 13-15, Patau syndrome) 60 Pierre-Robin syndrome 57 Moebius syndrome 55 Trisomy 18 (Edwards syndrome) 52 Norrie disease 38 Leber congenital amaurosis 35 Chromosome 18, Ring 18 31 Aicardi syndrome 29 Alstrom syndrome 27 Pfieffer syndrome 27 Treacher Collins syndrome 27 Waardenburg syndrome 27 Marshall syndrome 25 Refsum syndrome 21 Cri du chat syndrome (Chromosome 5p- synd) 16 Bardet-Biedl syndrome (Laurence Moon-Biedl) 15 Hurler syndrome (MPS I-H) 15 Crouzon syndrome (Craniofacial Dysotosis) 13 NF1 - Neurofibromatosis (von Recklinghausen dis) 13 Kniest Dysplasia 12 Turner syndrome 11 Usher III syndrome 10 Cockayne syndrome 9 Apert syndrome/Acrocephalosyndactyly, Type 1 8 Leigh Disease 8 Alport syndrome 6 Monosomy 10p 6 NF2 - Bilateral Acoustic Neurofibromatosis 6 Batten disease 5 Kearns-Sayre syndrome 5 Klippel-Feil sequence 5 Hereditary Syndromes and Disorders Count Prader-Willi 5 Sturge-Weber syndrome 5 Marfan syndrome 3 Hand-Schuller-Christian (Histiocytosis X) 2 Hunter Syndrome (MPS II) 2 Maroteaux-Lamy syndrome (MPS VI) 2 Morquio syndrome (MPS IV-B) 2 Optico-Cochleo-Dentate Degeneration 2 Smith-Lemli-Opitz (SLO) syndrome 2 Wildervanck syndrome 2 Herpes-Zoster (or Hunt) 1 Vogt-Koyanagi-Harada -

Involvements of Hyperhomocysteinemia in Neurological Disorders
H OH metabolites OH Review Involvements of Hyperhomocysteinemia in Neurological Disorders Marika Cordaro 1,† , Rosalba Siracusa 2,† , Roberta Fusco 2 , Salvatore Cuzzocrea 2,3,* , Rosanna Di Paola 2,* and Daniela Impellizzeri 2 1 Department of Biomedical, Dental and Morphological and Functional Imaging, University of Messina, Via Consolare Valeria, 98125 Messina, Italy; [email protected] 2 Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina, 98166 Messina, Italy; [email protected] (R.S.); [email protected] (R.F.); [email protected] (D.I.) 3 Department of Pharmacological and Physiological Science, Saint Louis University School of Medicine, Saint Louis, MO 63104, USA * Correspondence: [email protected] (S.C.); [email protected] (R.D.P.); Tel.: +39-090-6765208 (S.C. & R.D.P.) † The authors equally contributed to the review. Abstract: Homocysteine (HCY), a physiological amino acid formed when proteins break down, leads to a pathological condition called hyperhomocysteinemia (HHCY), when it is over a definite limit. It is well known that an increase in HCY levels in blood, can contribute to arterial damage and several cardiovascular disease, but the knowledge about the relationship between HCY and brain disorders is very poor. Recent studies demonstrated that an alteration in HCY metabolism or a deficiency in folate or vitamin B12 can cause altered methylation and/or redox potentials, that leads to a modification on calcium influx in cells, or into an accumulation in amyloid and/or tau protein involving a cascade of events that culminate in apoptosis, and, in the worst conditions, neuronal death. The present review will thus summarize how much is known about the possible role of HHCY in neurodegenerative disease. -

Megalocornea Jeffrey Welder and Thomas a Oetting, MS, MD September 18, 2010
Megalocornea Jeffrey Welder and Thomas A Oetting, MS, MD September 18, 2010 Chief Complaint: Visual disturbance when changing positions. History of Present Illness: A 60-year-old man with a history of simple megalocornea presented to the Iowa City Veterans Administration Healthcare System eye clinic reporting visual disturbance while changing head position for several months. He noticed that his vision worsened with his head bent down. He previously had cataract surgery with an iris-sutured IOL due to the large size of his eye, which did not allow for placement of an anterior chamber intraocular lens (ACIOL) or scleral-fixated lens. Past Medical History: Megalocornea Medications: None Family History: No known history of megalocornea Social History: None contributory Ocular Exam: • Visual Acuity (with correction): • OD 20/100 (cause unknown) • OS 20/20 (with upright head position) • IOP: 18mmHg OD, 17mmHg OS • External Exam: normal OU • Pupils: No anisocoria and no relative afferent pupillary defect • Motility: Full OU. • Slit lamp exam: megalocornea (>13 mm in diameter) and with anterior mosaic dystrophy. Iris-sutured posterior chamber IOLs (PCIOLs), stable OD, but pseudophacodonesis OS with loose inferior suture evident. • Dilated funduscopic exam: Normal OU Clinical Course: The patient’s iris-sutured IOL had become loose (tilted and de-centered) in his large anterior chamber, despite several sutures that had been placed in the past, resulting now in visual disturbance with movement. FDA and IRB approval was obtained to place an Artisan iris-clip IOL (Ophtec®). He was taken to the OR where his existing IOL was removed using Duet forceps and scissors. The Artisan IOL was placed using enclavation iris forceps. -

Blepharophimosis, Ptosis, and Epicanthus Inversus Syndrome
Blepharophimosis, ptosis, and epicanthus inversus syndrome Case Report A rare case of adult-onset blepharophimosis, ptosis, and epicanthus inversus syndrome: Case report Mahesha S1, Shruthi Bhimalli2, Manoj Y Bhat2 From 1Chief Medical Officer, 2Fellow in IOL, Department of Cataract and Trauma, Sankara Eye Hospital, Harakere, Shimoga, Karnataka, India Correspondence to: Dr. Shruthi Bhimalli, Department of Cataract and Trauma, Sankara Eye Hospital, Harakere, Shimoga - 577202, Karnataka, India. E-mail: [email protected] Received - 02 June 2019 Initial Review - 24 June 2019 Accepted - 25 July 2019 ABSTRACT Blepharophimosis, ptosis, and epicanthus inversus syndrome (BPES) is a rare genetic condition caused by a mutation in the FOXL2 gene and it is inherited in an autosomal dominant pattern. Identification and diagnosis of BPES syndrome by an ophthalmologist are relatively easy, based on the characteristic ocular manifestations. The most common age group at the time of diagnosis is 4 to 8 years. Here, we present an unusual case of BPES in a patient who presented with the syndrome at the age of 52 years. There is a need for increased awareness about this condition among ophthalmologists as early diagnosis is the key factor in preventing long term complications. Keywords: Blepharophimosis, Epicanthus inversus syndrome, Ptosis. lepharophimosis, ptosis, and epicanthus inversus action, epicanthus inversus and telecanthus (Fig. 1). On acuity syndrome (BPES) is a genetic condition associated testing, his best vision was ‘finger counting close to face’ in his Bwith mutations in the Fork head Box L2 (FOXL2) gene. right eye and ‘finger counting at one meter’ in his left eye. He had The syndrome is inherited in an autosomal dominant pattern, with corneal ectasia with scarring and vascularization (Fig. -

Prominent and Regressive Brain Developmental Disorders Associated with Nance-Horan Syndrome
brain sciences Article Prominent and Regressive Brain Developmental Disorders Associated with Nance-Horan Syndrome Celeste Casto 1,†, Valeria Dipasquale 1,†, Ida Ceravolo 2, Antonella Gambadauro 1, Emanuela Aliberto 3, Karol Galletta 4, Francesca Granata 4, Giorgia Ceravolo 1, Emanuela Falzia 5, Antonella Riva 6 , Gianluca Piccolo 6, Maria Concetta Cutrupi 1, Pasquale Striano 6,7 , Andrea Accogli 7,8, Federico Zara 7,8, Gabriella Di Rosa 9, Eloisa Gitto 10, Elisa Calì 11, Stephanie Efthymiou 11 , Vincenzo Salpietro 6,7,11,*, Henry Houlden 11 and Roberto Chimenz 12 1 Department of Human Pathology in Adult and Developmental Age “Gaetano Barresi”, Unit of Emergency Pediatric, University of Messina, Via Consolare Valeria 1, 98125 Messina, Italy; [email protected] (C.C.); [email protected] (V.D.); [email protected] (A.G.); [email protected] (G.C.); [email protected] (M.C.C.) 2 Unit of Ophthalmology, Department of Clinical and Experimental Medicine, University of Messina, Via Consolare Valeria 1, 98125 Messina, Italy; [email protected] 3 Casa di Cura la Madonnina, Via Quadronno 29, 20122 Milano, Italy; [email protected] 4 Department of Biomedical, Dental Science and Morphological and Functional Images, Neuroradiology Unit, University of Messina, Via Consolare Valeria 1, 98125 Messina, Italy; [email protected] (K.G.); [email protected] (F.G.) 5 Azienza Ospedaliera di Cosenza, Via San Martino, 87100 Cosenza, Italy; [email protected] 6 Pediatric Neurology and Muscular Diseases Unit, -

Eyelid Conjunctival Tumors
EYELID &CONJUNCTIVAL TUMORS PHOTOGRAPHIC ATLAS Dr. Olivier Galatoire Dr. Christine Levy-Gabriel Dr. Mathieu Zmuda EYELID & CONJUNCTIVAL TUMORS 4 EYELID & CONJUNCTIVAL TUMORS Dear readers, All rights of translation, adaptation, or reproduction by any means are reserved in all countries. The reproduction or representation, in whole or in part and by any means, of any of the pages published in the present book without the prior written consent of the publisher, is prohibited and illegal and would constitute an infringement. Only reproductions strictly reserved for the private use of the copier and not intended for collective use, and short analyses and quotations justified by the illustrative or scientific nature of the work in which they are incorporated, are authorized (Law of March 11, 1957 art. 40 and 41 and Criminal Code art. 425). EYELID & CONJUNCTIVAL TUMORS EYELID & CONJUNCTIVAL TUMORS 5 6 EYELID & CONJUNCTIVAL TUMORS Foreword Dr. Serge Morax I am honored to introduce this Photographic Atlas of palpebral and conjunctival tumors,which is the culmination of the close collaboration between Drs. Olivier Galatoire and Mathieu Zmuda of the A. de Rothschild Ophthalmological Foundation and Dr. Christine Levy-Gabriel of the Curie Institute. The subject is now of unquestionable importance and evidently of great interest to Ophthalmologists, whether they are orbital- palpebral specialists or not. Indeed, errors or delays in the diagnosis of tumor pathologies are relatively common and the consequences can be serious in the case of malignant tumors, especially carcinomas. Swift diagnosis and anatomopathological confirmation will lead to a treatment, discussed in multidisciplinary team meetings, ranging from surgery to radiotherapy. -

CHARGE Syndrome
orphananesthesia Anaesthesia recommendations for CHARGE syndrome Disease name: CHARGE syndrome ICD 10: Q87.8 Synonyms: CHARGE association; Hall-Hittner syndrome Disease summary: CHARGE syndrome was initially defined as a non-random association of anomalies: - Coloboma - Heart defect - Atresia choanae (choanal atresia) - Retarded growth and development - Genital hypoplasia - Ear anomalies/deafness In 1998, an expert group defined the major (the classical 4C´s: Choanal atresia, Coloboma, Characteristic ear and Cranial nerve anomalies) and minor criteria of CHARGE syndrome [1]. In 2004, mutations in the CHD7 gene were identified as the major cause. The inheritance pattern is autosomal dominant with variable expressivity. Almost all mutations occurs de novo, but parent-to-child transmission has occasionally been reported [2]. Clinical criteria for CHARGE syndrome [1] Major criteria: • Coloboma • Choanal Atresia • Cranial nerve anomalies • Abnormalities of the inner, middle, or external ear Minor criteria: • Cardiaovascular malformations • Genital hypoplasia or delayed pubertal development • Cleft lip and/or palate • Tracheoesophageal defects • Distinctive CHARGE facies • Growth retardation • Developmental delay Occasional: • Renal anomalies: duplex system, vesicoureteric reflux • Spinal anomalies: scoliosis, osteoporosis • Hand anomalies 1 • Neck/shoulder anomalies • Immune system disorders Individuals with all four major characteristics or three major and three minor characteristics are highly likely to have CHARGE syndrome [1]. CHARGE syndrome