XVL-Organo-Derivatives of Bismuth. Part V. the Stability of Halogen, Cyano-, and Thiocyano- Derivatives of Tertiary Aromatic Bismuthines
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cisco Controlled Substances Specification Revision B6 EDCS-661823 Page 1
Cisco Controlled Substances Specification Revision B6 EDCS-661823 Page 1 Doc Number: EDCS-661823 Last Revision Date: 04/30/2021 Policy Owner: Jack Allen Joe Johnson Policy Owner’s Org: Supply Chain Transformation: Sustainability Legal Market Access: Environmental Affairs Next Review Date: Upon changes in applicable global market access requirements Cisco Controlled Substances Specification Revision B6 Cisco Systems, Inc Page 1 of 35 CISCO CONFIDENTIAL All printed copies and duplicate soft copies are considered un-Controlled copies and the original on-line version should be referred for latest version Cisco Controlled Substances Specification Revision B6 EDCS-661823 Page 2 – Table of Contents – Executive Summary .................................................................................................................................... 3 Scope ........................................................................................................................................................... 3 Policy Statement ......................................................................................................................................... 3 1.1. Substances Restricted in Products ........................................................................................................... 3 1.2. Assessment Substances ............................................................................................................................ 7 1.3. Manufacturing Controlled Substances & Consumable Materials .......................................................... -

Hazardous Material Inventory Statement
City of Brooklyn Park FIRE DEPARTMENT 5200 - 85th Avenue North Brooklyn Park MN 55443 Phone: (763)493-8020 Fax: (763) 493-8391 Hazardous Materials Inventory Statement Users Guide A separate inventory statement shall be provided for each building. An amended inventory statement shall be provided within 30 days of the storage of any hazardous materials or plastics that changes or adds a hazard class or which is sufficient in quantity to cause an increase in the quantity which exceeds 5 percent for any hazard class. The hazardous materials inventory statement shall list by hazard class categories. Each grouping shall provide the following information for each hazardous material listed for that group including a total quantity for each group of hazard class. 1. Hazard class. (See attached Hazardous Materials Categories Listing) 2. Common or trade name. 3. Chemical Abstract Service Number (CAS number) found in 29 Code of Federal Regulations (C.F.R.). 4. Whether the material is pure or a mixture, and whether the material is a solid, liquid or gas 5. Maximum aggregate quantity stored at any one time. 6. Maximum aggregate quantity In-Use (Open to atmosphere) at any one time. 7. Maximum aggregate quantity In-Use (Closed to atmosphere) at any one time. 8. Storage conditions related to the storage type, high-pile, encapsulated, non-encapsulated. Attached is a listing of categories that all materials need to be organized to. Definitions of these categories are also attached for your use. At the end of this packet are blank forms for completing this project. For questions regarding Hazardous Materials Inventory Statement contact the Fire Department at 763-493-8020. -

Search for New Three-, Four-Component Petasis, Passerini
J. Chem. Chem. Eng. 8 (2014) 428-432 D DAVID PUBLISHING Search for New Three-, Four-Component Petasis, Passerini, Hantzsch, Kabachnic-Fields, Ugi Reactions with Organic Compounds of Phosphorus, Arsenic, Antimony and Bismuth Aibassov Erkin Zhakenovich*, Yemelyanova Valentina Stepanovna, Shakieva Tatyana Vladimirovna, Tussupbaev Nessipbay Kuandykovich and Blagikh Evgeniy Vladimirovich Research Institute of New Chemical Technologies and Materials, Kazakh National University Al-Farabi, Almaty 005012, Kazakhstan Received: November 29, 2013 / Accepted: December 18, 2013 / Published: April 25, 2014. Abstract: It is discovered a new three-, four-component Petasis, Passerini, Hantzsch, Kabachnic-Fields, Ugi reactions with of arsine, stibine and bismuthine in organometallic chemistry. Modifications were replaced to a nitrogen atom of classical reactions of atoms of phosphorus, arsenic, antimony and bismuth. It has been proposed a new mechanism for possible reactions. Key words: Petasis, Passerini, Hantzsch, Kabachnic-Fields, Ugi reactions, phosphorus, arsenic, antimony, bismuth. 1. Introduction four-component reactions Petasis, Passerini, Hantzsch, Kabachnic-Fields, Ugi reactions with organic In recent years, it has been actively investigated compounds of phosphorus, arsenic, antimony and nucleophilic reaction organilgalogenidami phosphine bismuth. and with electrophilic geterilalkenami allowing to obtain primary, secondary and tertiary phosphines, 2. Theory selectively and with high yield. Most of these It is known that the Petasis reaction is a MCR reactions are realized under mild conditions at a (multi component reaction) that enables the pressure atmlosfernom phosphine. preparation of amines and their derivatives such as Although the chemistry of organophosphorus α-amino acids. compounds devoted a considerable number of monographs, but the reaction of organic compounds of arsenic, antimony and bismuth are very limited. -

2019 Minnesota Chemicals of High Concern List
Minnesota Department of Health, Chemicals of High Concern List, 2019 Persistent, Bioaccumulative, Toxic (PBT) or very Persistent, very High Production CAS Bioaccumulative Use Example(s) and/or Volume (HPV) Number Chemical Name Health Endpoint(s) (vPvB) Source(s) Chemical Class Chemical1 Maine (CA Prop 65; IARC; IRIS; NTP Wood and textiles finishes, Cancer, Respiratory 11th ROC); WA Appen1; WA CHCC; disinfection, tissue 50-00-0 Formaldehyde x system, Eye irritant Minnesota HRV; Minnesota RAA preservative Gastrointestinal Minnesota HRL Contaminant 50-00-0 Formaldehyde (in water) system EU Category 1 Endocrine disruptor pesticide 50-29-3 DDT, technical, p,p'DDT Endocrine system Maine (CA Prop 65; IARC; IRIS; NTP PAH (chem-class) 11th ROC; OSPAR Chemicals of Concern; EuC Endocrine Disruptor Cancer, Endocrine Priority List; EPA Final PBT Rule for 50-32-8 Benzo(a)pyrene x x system TRI; EPA Priority PBT); Oregon P3 List; WA Appen1; Minnesota HRV WA Appen1; Minnesota HRL Dyes and diaminophenol mfg, wood preservation, 51-28-5 2,4-Dinitrophenol Eyes pesticide, pharmaceutical Maine (CA Prop 65; IARC; NTP 11th Preparation of amino resins, 51-79-6 Urethane (Ethyl carbamate) Cancer, Development ROC); WA Appen1 solubilizer, chemical intermediate Maine (CA Prop 65; IARC; IRIS; NTP Research; PAH (chem-class) 11th ROC; EPA Final PBT Rule for 53-70-3 Dibenzo(a,h)anthracene Cancer x TRI; WA PBT List; OSPAR Chemicals of Concern); WA Appen1; Oregon P3 List Maine (CA Prop 65; NTP 11th ROC); Research 53-96-3 2-Acetylaminofluorene Cancer WA Appen1 Maine (CA Prop 65; IARC; IRIS; NTP Lubricant, antioxidant, 55-18-5 N-Nitrosodiethylamine Cancer 11th ROC); WA Appen1 plastics stabilizer Maine (CA Prop 65; IRIS; NTP 11th Pesticide (EPA reg. -

PHOTODISSOCIATION DYNAMICS of GROUP V HYDRIDES By
PHOTODISSOCIATION DYNAMICS OF GROUP V HYDRIDES by William P. Schroeder A Dissertation Presented to the FACULTY OF THE USC GRADUATE SCHOOL UNIVERSITY OF SOUTHERN CALIFORNIA In Partial Fulfillment of the Requirements for the Degree DOCTOR OF PHILOSOPHY (CHEMISTRY) May 2013 Copyright 2013 William P. Schroeder i This dissertation is dedicated to my favorite lab partner, life partner, and best friend, Christi A. Schroeder. !" ii Table of Contents Abstract 1 Chapter 1 Relativistic Effects in Chemistry 2 1.1 Introduction 2 1.2 Relativistic Effects 3 1.2.1 Qualitative Effects 3 1.2.2 Early Relativistic Quantum Mechanics 8 1.2.3 Dirac Equation 11 1.2.4 Relativistic Many-Body Problem 17 1.3 The Potential Energy Surface (PES) 19 1.4 Photodissociation Dynamics 25 1.5 Chapter 1 References 28 Chapter 2 Experimental Methods 30 2.1 Introduction 30 2.2 Time-of-Flight Spectroscopy 30 2.2.1 Hydrogen Time-of-Flight Spectroscopy 30 2.2.2 High-n Rydberg Time-of-Flight Spectroscopy 31 2.3 Experimental Setup and Details 32 2.3.1 Vacuum Chamber and System 32 2.3.2 Laser Systems 36 2.3.3 Electronic Control and Detection 37 2.3.4 System Alignment 38 2.4 Data Collection and Processing 39 2.5 Chapter 2 References 42 Chapter 3 The UV Photodissociation Dynamics of Arsine (AsH3) 43 3.1 Introduction 43 3.2 Experimental 50 3.3 Results 51 3.4 Discussion 55 3.4.2 AsH2 internal excitations 58 3.4.3 Secondary Photolysis: AsH2 # AsH + H 62 3.5 Conclusions 64 3.6 Chapter 3 References 67 Chapter 4 Supplemental Data, Design Considerations and Safety 70 4.1 Introduction 71 iii -

The Radiochemistry of Bismuth
NAS-NS-3061 RA OFBISMUTH NUCLEAR SCIENCE SERIES National Academy of Sciences - National Research Council Published by Technical Information Center ENERGY RESEARCH AND DEVELOPMENT ADMINISTRATION COMMITTEE ON NUCLEAR SCIENCE John Huizenga, Chairman, Nuclear WrUcture Re=arch Laboratory Thomas A. Tombrello, Vice Chairman, California institute of T=hnology C. K. Reed, Executive Secretary,Netional Academy of Sciences Lowell M. Bollinger, Argonne Nationel Laboratow Peggy Dyer, UnivarsiW of Washington Rusaall Heath, Aerojet Nuclear Co., Inc. Roy K. Middlaton, University of Pennsylvania 1: Lon Morgan, Columbie Scientific Industries G. Davis O’Kelley, Oek Ridge National Laboratow G. C. Phillips, Rice University Henry N. Wagner, Jr., The Johns Hopkins Medial Institutions Joseph Wen~, Brookhaven National Laboratory Sheldon’ Wolff, University of California Chien-Shiung Wu, Columbia Univar?@ Alexander Zuckar, Oak Ridga National Laborato~ Liaison Members William S. Rodney, National science Foundation George L. ROWS, Energy Research and Development Admini-ration SUBCOMMITTEE ON RAD1OCHEMISTRY G. Davis O’Kelley, Chairmsrr, Oak Ridge National Laboratory Glen E. Gordon, UnivwsiW of Maryler& ‘“- ,-. Rolfa H. Hw*r, Rutgers Univemity John A. Miskel, Lawrence Livermore LaboratoW Harold A. O’Brien, Jr., Los Alamos Scientific Laboratory Richard W. Perkins. Bettafle Pacific Northwest Laboratories Andrew F. Stehney, Argonne National Laboratory Kurt Wotfsbarg, Los Alanros Scientific Laboratow LiaisonMembers ~ John L. Burnatte, Energy Research and Davelopmant Administration FTed Findeis, National Scienca Foundation i.,.~.. Radiochemistry of Bismuth Kashinath S. Bhatki Tata Instituteof Fundamental Research Homi Bhabha Road, Bombay 400005 and Bhabha Atomic ResearchC-entre Trornbay,Bombay 400085 (India) Prepared for Subcommittee on Radiochemistry National Academy of Sciences - Natiorial Research Council IssuanceDate:September 1977 Published by Technical 1nform,ation center ENERGY RESEARCH AND DEVELOPMENT ADMINISTRATION Price$4.75.Availablefrom: NationalTechnicalInformationservice U. -

Structural Evidence for Pnictogen-Centered Lewis Acidity in Cationic Platinum-Stibine Complexes Featuring Pendent Amino Or Ammonium Groups †
molecules Article Structural Evidence for Pnictogen-Centered Lewis Acidity in Cationic Platinum-Stibine Complexes Featuring Pendent Amino or Ammonium Groups † Roberta R. Rodrigues and François P. Gabbaï * Department of Chemistry, Texas A&M University, College Station, TX 77843-3255, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-979-862-2070 † In memory of Alan H. Cowley, a most inspiring Ph.D. advisor, and a great friend. Abstract: As part of our continuing interest in the chemistry of cationic antimony Lewis acids as ligands for late transition metals, we have now investigated the synthesis of platinum complexes featuring a triarylstibine ligand substituted by an o-[(dimethylamino)methyl]phenyl group referred N N to as Ar . More specifically, we describe the synthesis of the amino stibine ligand Ph2SbAr (L) and its platinum dichloride complex [LPtCl]Cl which exists as a chloride salt and which shows weak coordination of the amino group to the antimony center. We also report the conversion of [LPtCl]Cl 3+ into a tricationic complex [LHPt(SMe2)] which has been isolated as a tris-triflate salt after reaction of [LPtCl]Cl with SMe2, HOTf and AgOTf. Finally, we show that [LHPt(SMe2)][OTf]3 acts as a catalyst for the cyclization of 2-allyl-2-(2-propynyl)malonate. Citation: Rodrigues, R.R.; Gabbaï, Keywords: pnictogen bonding; platinum; catalysis F.P. Structural Evidence for Pnictogen-Centered Lewis Acidity in Cationic Platinum-Stibine Complexes Featuring Pendent Amino or 1. Introduction Ammonium Groups . Molecules 2021, The chemistry of stibines is attracted a renewed interest because of applications in 26, 1985. -
Sorted by CAS Number
This document is made available electronically by the Minnesota Legislative Reference Library as part of an ongoing digital archiving project. http://www.leg.state.mn.us/lrl/lrl.asp Minnesota Department of Health, Chemicals of High Concern list, July 1, 2010 (updated October 7, 2010) Persistent, Bioaccumulative, Toxic or very Persistent, very HPV (2006 and 3 of 4 CAS Number Chemical Name Health endpoint(s) Bioaccumulative Source(s) Use example(s) or class years)1 50-00-0 Formaldehyde (in water) Gastrointestinal system Minnesota HRL Contaminant Maine (CA Prop 65; IARC; IRIS; NTP 11th ROC); WA Appen1; Cancer, Respiratory system, WA CHCC; Minnesota HRV; Wood and textiles finishes, disinfection, 50-00-0 Formaldehyde Eye irritant Minnesota RAA tissue preservative x Maine (CA Prop 65; IARC; IRIS; NTP 11th ROC; OSPAR Chemicals of Concern; EU Endocrine Disruptor; EPA Final PBT Rule for TRI; EPA Priority PBT); Oregon P3 List; WA Appen1; Minnesota 50-32-8 Benzo(a)pyrene Cancer, Endocrine system x HRV Combustion by product, research Dyes and diaminophenol mfg, wood 51-28-5 2,4-Dinitrophenol Eyes WA Appen1; Minnesota HRL preservation, pesticide, pharmaceutical Maine (CA Prop 65; IARC); WA 51-75-2 Nitrogen mustard (Mechlorethamine) Cancer, Development Appen1 Warfare agent, research Maine (CA Prop 65; IARC; NTP Preparation of amino resins, solubilizer, 51-79-6 Urethane (Ethyl carbamate) Cancer, Development 11th ROC); WA Appen1 chemical intermediate Maine (CA Prop 65; IARC; IRIS; NTP 11th ROC; EPA Final PBT Rule for TRI; WA PBT List; OSPAR Chemicals -

Coordination Chemistry of the Main Group Elements with Phosphine, Arsine and Stibine Ligands
Coordination Chemistry Reviews 260 (2014) 65–115 Contents lists available at ScienceDirect Coordination Chemistry Reviews jo urnal homepage: www.elsevier.com/locate/ccr Review Coordination chemistry of the main group elements with phosphine, arsine and stibine ligands ∗ Jennifer Burt, William Levason , Gillian Reid School of Chemistry, University of Southampton, Southampton SO17 1BJ, UK Contents 1. Introduction . 66 2. Bonding . 66 3. Characterisation techniques . 67 4. s-Block complexes . 68 5. Group 12 . 68 5.1. Zinc . 69 5.2. Cadmium . 70 5.3. Mercury . 71 6. Group 13 . 74 6.1. Boron . 74 6.2. Aluminium. 76 6.3. Gallium . 81 6.4. Indium . 87 6.5. Thallium . 93 7. Group 14 . 94 7.1. Silicon. 94 7.2. Germanium . 94 7.3. Tin . 98 7.4. Lead . 102 8. Group 15 . 102 8.1. Phosphorus . 103 8.2. Arsenic . 103 8.3. Antimony . 104 8.4. Bismuth . 106 9. Group 16 . 108 10. Applications and outlook . .. -

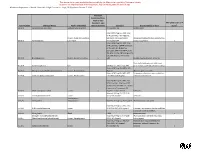
Chemical Inventory Packet / Calarp Lists / HFD / Dmg 2004 Table 1
HAYWARD FIRE DEPARTMENT CHEMICAL INVENTORY WORKSHEET To prepare a chemical inventory based on the Hayward Fire Code, you are required to report the quantities of chemicals found in the facility, separated by Hazard Category, and by location. You are also to classify the chemicals as to storage and manner of use. Handling and storage requirements are determined by these factors: the nature of the chemical or its hazard; the location; the manner of use or storage; and the quantity involved in each particular manner of use or storage. A Control Area is a building, a portion of the building, or an outside area where chemicals are allowed to be stored, dispensed, used or handled subject to certain conditions and requirements. This packet consists of the following lists and the Chemical Inventory Worksheet form: 1. Hazardous Materials Hazard Categories. This is the list printed on goldenrod paper. Based on the Uniform Fire Code, this is the classification of chemicals by hazard categories. The different hazard categories are defined and examples are provided for each. Most chemicals exhibit more than one hazard, and all hazards should be considered when preparing your chemical inventory. 2. Tables of Regulated Substances Under the CalARP Program. Printed on pink paper, these are the lists of flammable and toxic substances that, if present at or above certain threshold quantities, will require the preparation of a Risk Management Plan 3. Federal List of Extremely Hazardous Substances (EHS). This list is printed on blue paper. These are the substances that the federal government classifies as extremely hazardous substances. The Hayward Fire Department has included “CalARP-Listed or EHS-Listed Chemicals” as a separate Hazard Category. -

GLOSSARY of CLASS NAMES of ORGANIC COMPOUNDS and REACTIVE INTERMEDIATES BASED on STRUCTURE (IUPAC Recommendations 1995)
Pure &App/. Chem., Vol. 67, Nos 819, pp. 1307-1375, 1995. Printed in Great Britain. (B 1995 IUPAC INTERNATIONAL UNION OF PURE AND APPLIED CHEMISTRY ORGANIC CHEMISTRY DMSION COMMISSION ON NOMENCLATURE OF ORGANIC CHEMISTRY (III. 1) COMMISSION ON PHYSICAL ORGANIC CHEMISTRY (III.2) GLOSSARY OF CLASS NAMES OF ORGANIC COMPOUNDS AND REACTIVE INTERMEDIATES BASED ON STRUCTURE (IUPAC Recommendations 1995) Prepared for publication by G.P. MOSS, P.A.S. SMITH and D. TAVERNIER Cornpodtion of the ZZZ.1 & 111.2 Working Party (1980-1994): H.J.T. Bos, A.J. Boulton, E.W. Godly, P. Griinanger, A.D. McNaught, G.P. Moss, R. PanicO, J. Rigaudy, P.A.S. Smith (Conwnorfiom ZZZ, I), J.H. Stocker, D. Tavernier, R.A.Y. Jones, J. March, J.M. McBnde, P. Miiller (Conwnorfim 111.2). Membership of the Commission on Nomenclature of Organic Chemistry during the preparation of this document (1980-1994) was as follows: Titular Members: 0. Achmatowicz (Poland) 1979-1987; H. J. T. Bos (Netherlands) 1987- , Vice-Chairman, 1991- ; J. R. Bull (Republic of South Africa) 1987-1993; H. A. Favre (Canada) 1989- , Chairman, 1991- ; P. M. Giles, Jr. (USA) 1989- ; E. W. Godly (UK) 1987-1993. Secretary, 1989-1993 ; D. Hellwinkel (Federal Republic of Germany) 1979-1987, Vice- Chairman, 1981-1987; B. J. Herold (Portugal) 1994- ; K. Hirayama (Japan) 1975-1983; M. V. KisakUrck (Switizrland) 1994- ; A. D. McNaught (UK) 1979-1987; G. P. Moss (UK) 1977- 1987, Chairinan, 1981- 1987, Vice-Chainnun, 1979-1981; R. Panico (Francc) 1981-1991, Vice- Chairman, 1989-1991; W. H. Powell (USA) Secretary, 1979-1989; J. -
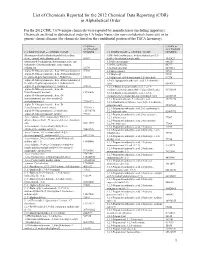
List of Chemicals Reported for the 2012 Chemical Data Reporting (CDR) in Alphabetical Order
List of Chemicals Reported for the 2012 Chemical Data Reporting (CDR) in Alphabetical Order For the 2012 CDR, 7,674 unique chemicals were reported by manufacturers (including importers). Chemicals are listed in alphabetical order by CA Index Name (for non-confidential chemicals) or by generic chemical name (for chemicals listed on the confidential portion of the TSCA Inventory). CASRN or CASRN or ACCESSION ACCESSION CA INDEX NAME or GENERIC NAME NUMBER CA INDEX NAME or GENERIC NAME NUMBER (Benzoquinolinyl)-(alkylimidazolyl)indenedione 1(3H)-Isobenzofuranone, 6-(dimethylamino)-3,3- deriv., compd. with alkanoic acid 56907 bis[4-( dimethylamino)phenyl]- 1552427 (Substituted-5-sulfophenyl heteromonocycle) azo- 1,10-Decanediamine 646253 (substituted 2-hydroxyphenyl), iron complex, 1,10-Phenanthroline 66717 sodium salt 20736 1,12-Dodecanediol 5675514 .alpha.-D-Glucopyranoside, .beta.-D-fructofuranosyl 57501 1,1'-Bicyclohexyl 92513 .alpha.-D-Glucopyranoside, .beta.-D-fructofuranosyl 1,1'-Biphenyl 92524 O-.alpha.-D-galactopyranosyl-( 1.fwdarw.6)- 512696 1,1'-Biphenyl, 4,4'-diisocyanato-3,3'-dimethyl- 91974 .alpha.-D-Glucopyranoside, .beta.-D-fructofuranosyl 1,1-Cyclopropanedicarboxylic acid, 1,1-dimethyl O-.alpha.-D-galactopyranosyl-( 1.fwdarw.6)-O- ester 6914712 .alpha.-D-g alactopyranosyl-(1.fwdarw.6)- 470553 1,2,3,4-Butanetetracarboxylic acid, 1,2,3,4- .alpha.-D-Glucopyranoside, .beta.-D- tetrakis(1,2,2,6,6-pentamethyl-4 -piperidinyl) ester 91788839 fructofuranosyl, benzoate 12738646 1,2,3,4-Butanetetracarboxylic acid, 1,2,3,4- .alpha.-D-Glucopyranoside,