Opinion on Bismuth Citrate ______
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cisco Controlled Substances Specification Revision B6 EDCS-661823 Page 1
Cisco Controlled Substances Specification Revision B6 EDCS-661823 Page 1 Doc Number: EDCS-661823 Last Revision Date: 04/30/2021 Policy Owner: Jack Allen Joe Johnson Policy Owner’s Org: Supply Chain Transformation: Sustainability Legal Market Access: Environmental Affairs Next Review Date: Upon changes in applicable global market access requirements Cisco Controlled Substances Specification Revision B6 Cisco Systems, Inc Page 1 of 35 CISCO CONFIDENTIAL All printed copies and duplicate soft copies are considered un-Controlled copies and the original on-line version should be referred for latest version Cisco Controlled Substances Specification Revision B6 EDCS-661823 Page 2 – Table of Contents – Executive Summary .................................................................................................................................... 3 Scope ........................................................................................................................................................... 3 Policy Statement ......................................................................................................................................... 3 1.1. Substances Restricted in Products ........................................................................................................... 3 1.2. Assessment Substances ............................................................................................................................ 7 1.3. Manufacturing Controlled Substances & Consumable Materials .......................................................... -

The Radiochemistry of Bismuth
NAS-NS-3061 RA OFBISMUTH NUCLEAR SCIENCE SERIES National Academy of Sciences - National Research Council Published by Technical Information Center ENERGY RESEARCH AND DEVELOPMENT ADMINISTRATION COMMITTEE ON NUCLEAR SCIENCE John Huizenga, Chairman, Nuclear WrUcture Re=arch Laboratory Thomas A. Tombrello, Vice Chairman, California institute of T=hnology C. K. Reed, Executive Secretary,Netional Academy of Sciences Lowell M. Bollinger, Argonne Nationel Laboratow Peggy Dyer, UnivarsiW of Washington Rusaall Heath, Aerojet Nuclear Co., Inc. Roy K. Middlaton, University of Pennsylvania 1: Lon Morgan, Columbie Scientific Industries G. Davis O’Kelley, Oek Ridge National Laboratow G. C. Phillips, Rice University Henry N. Wagner, Jr., The Johns Hopkins Medial Institutions Joseph Wen~, Brookhaven National Laboratory Sheldon’ Wolff, University of California Chien-Shiung Wu, Columbia Univar?@ Alexander Zuckar, Oak Ridga National Laborato~ Liaison Members William S. Rodney, National science Foundation George L. ROWS, Energy Research and Development Admini-ration SUBCOMMITTEE ON RAD1OCHEMISTRY G. Davis O’Kelley, Chairmsrr, Oak Ridge National Laboratory Glen E. Gordon, UnivwsiW of Maryler& ‘“- ,-. Rolfa H. Hw*r, Rutgers Univemity John A. Miskel, Lawrence Livermore LaboratoW Harold A. O’Brien, Jr., Los Alamos Scientific Laboratory Richard W. Perkins. Bettafle Pacific Northwest Laboratories Andrew F. Stehney, Argonne National Laboratory Kurt Wotfsbarg, Los Alanros Scientific Laboratow LiaisonMembers ~ John L. Burnatte, Energy Research and Davelopmant Administration FTed Findeis, National Scienca Foundation i.,.~.. Radiochemistry of Bismuth Kashinath S. Bhatki Tata Instituteof Fundamental Research Homi Bhabha Road, Bombay 400005 and Bhabha Atomic ResearchC-entre Trornbay,Bombay 400085 (India) Prepared for Subcommittee on Radiochemistry National Academy of Sciences - Natiorial Research Council IssuanceDate:September 1977 Published by Technical 1nform,ation center ENERGY RESEARCH AND DEVELOPMENT ADMINISTRATION Price$4.75.Availablefrom: NationalTechnicalInformationservice U. -

XVL-Organo-Derivatives of Bismuth. Part V. the Stability of Halogen, Cyano-, and Thiocyano- Derivatives of Tertiary Aromatic Bismuthines
View Article Online / Journal Homepage / Table of Contents for this issue ORGANO-DERIVATIVES OF BISMUTH. PART V. 91 XVL-Organo-derivatives of Bismuth. Part V. The Stability of Halogen, Cyano-, and Thiocyano- derivatives of Tertiary Aromatic Bismuthines. By FREDERICKCHALLENGER and JOHNFREDERICK WILKINSON. THE dihaloids of triphenylbismuthine show diminished stability with increase in the positive character of the halogen until, with iodine, the di-iodide, Ph,BiI,, has not been isolated but immediately decomposes, giving iodobenzene and diphenyliodobismuthine (Challenger and Allpress, T., 1915, 107, 21 ; Gillmeister, Ber., 1897, 30, 2843). As a result of work described in this and in previous communications, the relations existing between the dihaloids and similar derivatives may be summarised thus- 31. p. Temp. of Decomposition. Difluoride ........................ 159" 190-200" Dichloride ........................ 141" 150°, and slowly in boiling benzene. Dibromide ........................ 122" looo, and easily in boiling benzene. Di-iodide, not isolated, unstable, yielding Ph,BiI and PhI. Dicyanide, not isolated, unstable, yielding Ph,Bi.CN and Ph-CN. Dithiocyanate, not isolated, unstable, yielding Ph,Bi. SCN and Ph-SCN. The decomposition of the dihaloids may be represented by the equations- (i) Ph,BiX, = Ph,BiX + PhX or (ii) 2Ph,BiX, = Ph,Bi + PhBiX, + 2PhX and at a higher temperature, Published on 01 January 1922. Downloaded by University of Western Ontario 31/10/2014 11:40:05. (iii) 2Ph,Bi = 3Ph, + 2Bi. When triphenylbismuthine dichloride is treated with potassium fluoride in aqueous-alcoholic solution, triphenylbismuthine dijluoride, m. p. 159", is produced. This decomposes when heated, giving fluorobenzene, triphenylbismuthine ,diphenyl, and probably bismuth fluoride. Although no tetraphenylbismuthonium haloids have so far been obtained, the stability of the difluoride led us to hope that, by interaction with magnesium phenyl bromide, tetraphenyl- bismuthonium fluoride might possibly be formed, but only triphenyl- bismuthine was isolated. -
Bismuth(III) Triflate Catalyzed Allylation of Cyclic Acetals
Illinois Wesleyan University Digital Commons @ IWU Honors Projects Chemistry 2009 Green Chemistry Using Bismuth Compounds: Bismuth(III) Triflate Catalyzed Allylation of Cyclic Acetals Matthew J. Spafford, '09 Illinois Wesleyan University Follow this and additional works at: https://digitalcommons.iwu.edu/chem_honproj Part of the Physical Sciences and Mathematics Commons Recommended Citation Spafford, '09, Matthew .,J "Green Chemistry Using Bismuth Compounds: Bismuth(III) Triflate Catalyzed Allylation of Cyclic Acetals" (2009). Honors Projects. 33. https://digitalcommons.iwu.edu/chem_honproj/33 This Article is protected by copyright and/or related rights. It has been brought to you by Digital Commons @ IWU with permission from the rights-holder(s). You are free to use this material in any way that is permitted by the copyright and related rights legislation that applies to your use. For other uses you need to obtain permission from the rights-holder(s) directly, unless additional rights are indicated by a Creative Commons license in the record and/ or on the work itself. This material has been accepted for inclusion by faculty at Illinois Wesleyan University. For more information, please contact [email protected]. ©Copyright is owned by the author of this document. Green Chemistry Using Bismuth Compounds: Bismuth(III) TriflateCatalyzed Allylation of Cyclic Acetals Matthew J. Spafford Advisor: Dr. Ram Mohan Research Honors Senior Thesis Illinois Wesleyan University Spring 2009 Approval Page i Green Chemistry Using Bismuth Compounds: Bismuth(III) Triflate Catalyzed Allylation of Cyclic Acetals By Matthew Spafford 1. A PAPER SUBMITTED AS PART OF THE REQUIREMENTFOR RESEARCH HONORS IN CHEMISTRY Approved: Ram S. Mohan, Ph. D., Research Advisor ! Brian B. -
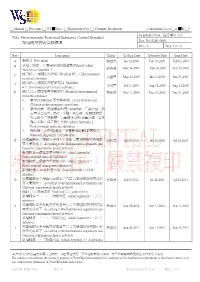
Environmental Restricted Substance Control Standard 環境限用物質管制
□ Manual □ Procedure □ WI █ Spec. □ Engineering Doc □ Company Regulation Confidential Level □A █B □C Established Dept: 品保單位 QA Title: Environmental Restricted Substance Control Standard Doc. No: SQS1-0001 環境限用物質管制標準 Rev.: K Page: 1 of 76 Rev. Description Editor Co-Sign Date Effective Date Issue Date A 新制訂 New initial 陳益光 Jan 12,2009 Feb 03,2009 Feb 03,2009 B 新增<附錄一>環境限用物質鹵素(Halgen) Added Halogen to Appendix 1 郭淑鳳 Mar 24,2009 Mar 26,2009 Mar 26,2009 C 修訂#7.1.1 環境限用物質 Modified #7.1.1 Environmental restricted substance 朱國華 May 22,2009 Jun 03,2009 Jun 03,2009 D 修訂#7.1.1 環境限用物質項目 Modified #7.1.1Environmental restricted substance 朱國華 Jul 24,2009 Aug 12,2009 Aug 12,2009 E 修訂 7.1.1 環境限用物質項目 Modified Environmental 林維彥 Nov 11,2009 Dec 01,2009 Dec 01,2009 restricted substance 1. 新增溴&氯為必要管制對象 Added Bromine and Chlorine as the necessary control item 2. 新增附錄一環境限用物質內容(鹵素、二氯化鈷、特 定苯並三氮唑、PVC、甲醛、氧化鈹、放射性物質、 特定鄰苯二甲酸鹽、氫氟碳化合物;全氟化碳、富馬 酸二甲酯、特定胺化合物) Added Appendix 1 Environmental restricted substance 3. 將附錄一(各物質編號、金屬換算系數)表頭刪除 Removed Appendix 1 of table title F 依據國際法令規範內容與客戶自訂之環境限用物質項目 林維彥 Jul 08,2010 Jul 19,2010 Jul 19,2010 要求更新如下 According to the International regulations and Customer requirements update as below 新增附錄一環境限用物質內容 Added Appendix 1 Environmental restricted substance 新增附錄二環境管理物質內容 Added Appendix 2 Environmental management substance 新增附錄三 ODS 物質內容 Added Appendix 3 ODS substance G 依據國際法令規範內容與客戶自訂之環境限用物質項目 林維彥 Jul 05,2011 Jul 14,2011 Jul 14,2011 要求更新如下 According to the International regulations and Customer requirements update as below 修訂 7.1.1 濃度限值 -

The Hydrothermal Chemistry of Bismuth and the Liquid Bismuth Collector Model
The Hydrothermal Chemistry of Bismuth and The Liquid Bismuth Collector Model A Thesis Submitted for the Degree of Doctor of Philosophy of the University of Adelaide January 2013 Cartoon by Nick D. Kim (www.lab-initio.com), Reproduced in accordance with free permissions for use in non profit, educational and research purposes. Blake Tooth Geology and Geophysics, School of Earth and Environmental Sciences, University of Adelaide Contents Abstract (vii) Declaration (ix) Acknowledgements (xi) Preface (xiii) Chapter 1: Introduction (1) 1. The uses of bismuth and economic aspects (3) 2. The Au-Bi Association (5) 3. Aims (7) 4. Thesis organisation (7) References (8) Chapter 2: Review of Bismuth Chemistry (11) 1. Properties of the Element (14) 2. Aqueous Bi(III) Chemistry (15) 3+ 2.1 Revised HKF Parameters of the Bi (aq) (15) 3. Hydrolysis of Bismuth (19) 3.1 Bismuth Aquo-Ion (19) 3.2 Mononuclear Species (20) 3.3 Polynuclear Species (21) 4. Bismuth in Chloride Bearing Solutions (24) 4.1 Acidic Chloride Solutions (24) 4.2 Disagreements Concerning BiCl52- and BiCl63- in the Speciation Scheme (25) 4.3 The Bronsted-Guggenheim-Scatchard Specific Ion Interaction (SIT) Model for Activity Coefficients (29) 4.4 Bismuth Hydrolysis in Chloride Solutions of Varying pH (32) 5. Bismuth in Sulfur Bearing Solutions (33) 6. Hydrothermal Chemistry of Bismuth (35) 7. Aqueous Bismuth Chemistry: Summary (37) References (38) iii Chapter 3: Bismuth speciation in hydrothermal fluids: An X-ray absorption spectroscopy and solubility study (45) Statement of Authorship (46) Abstract (47) 1. Introduction (47) 1.1 Bismuth in hydrothermal systems (47) 1.2 Aqueous chemistry of bismuth (48) 1.3 Hydrothermal Chemistry of Bismuth (49) 2. -

Nitrogen Group
Nitrogen group The nitrogen group is a periodic table group consisting of nitrogen ( N), phosphorus (P), arsenic ( As ), antimony ( Sb ), bismuth ( Bi ) and ununpentium ( Uup ) (unconfirmed. In modern IUPAC notation, it is called Group 15 . In the old IUPAC, it was called Group VB and Group VA , respectively (pronounced "group five B" and "group five A", because "V" is a Roman numeral. In the field of semiconductor physics, it You are using demo version You are using demo version is still universally called Group V . It is also collectively named the pnictogens .The "five" ("V") in the historical names comes from the fact that these elements have five valence electrons. Like other groups, the members of this family Please purchase full version from www.technocomsolutions.com Please purchase full version from www.technocomsolutions.com show patterns in its electron configuration, especially the outermost shells resulting in trends in chemical behavior Z Element No. of electrons/shell 7 nitrogen 2, 5 15 phosphorus 2, 8, 5 33 arsenic 2, 8, 18, 5 You are using demo version You are using demo version 51 antimony 2, 8, 18, 18, 5 83 bismuth 2, 8, 18, 32, 18, 5 Please purchase full version from www.technocomsolutions.com Please purchase full version from www.technocomsolutions.com 115 ununpentium 2, 8, 18, 32, 32, 18, 5 • This group has the defining characteristic that all the component elements have 5 electrons in their outermost shell, that is 2 electrons in the s subshell and 3 unpaired electrons in the p subshell. They are therefore 3 electrons short of filling their outermost electron shell in their non-ionized state. -

A Short Review on Biomedical Applications of Nanostructured Bismuth Oxide and Related Nanomaterials
materials Review A Short Review on Biomedical Applications of Nanostructured Bismuth Oxide and Related Nanomaterials Mattia Bartoli 1,2 , Pravin Jagdale 3 and Alberto Tagliaferro 1,2,* 1 Department of Applied Science and Technology, Politecnico di Torino, Corso Duca degli Abruzzi 24, 10129 Turin, Italy; [email protected] 2 Italian Institute of Technology, Via Livorno 60, 10144 Torino, Italy 3 Consorzio Interuniversitario Nazionale per la Scienza e Tecnologia dei Materiali (INSTM), Via G. Giusti 9, 50121 Florence, Italy; [email protected] * Correspondence: [email protected]; Tel.: +39-011-0907347 Received: 28 October 2020; Accepted: 18 November 2020; Published: 19 November 2020 Abstract: In this review, we reported the main achievements reached by using bismuth oxides and related materials for biological applications. We overviewed the complex chemical behavior of bismuth during the transformation of its compounds to oxide and bismuth oxide phase transitions. Afterward, we summarized the more relevant studies regrouped into three categories based on the use of bismuth species: (i) active drugs, (ii) diagnostic and (iii) theragnostic. We hope to provide a complete overview of the great potential of bismuth oxides in biological environments. Keywords: bismuth oxide; nanoparticles; radiopacity; chemotherapy; theragnostic 1. Introduction Recently, the concerns for human healthiness have risen day by day. Pharma industries and the research community have committed themselves to improve both the knowledge and on-field real application of newly designed drugs and protocols. Despite the numerous available established treatments, there is still an urgent need to develop new and innovative technologies that could help to delineate tumor margins, identify residual tumor cells and eventually eliminate them [1–3].