Development and Function of Plasmodesmata in Zygotes of Fucus Distichus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Algae & Marine Plants of Point Reyes
Algae & Marine Plants of Point Reyes Green Algae or Chlorophyta Genus/Species Common Name Acrosiphonia coalita Green rope, Tangled weed Blidingia minima Blidingia minima var. vexata Dwarf sea hair Bryopsis corticulans Cladophora columbiana Green tuft alga Codium fragile subsp. californicum Sea staghorn Codium setchellii Smooth spongy cushion, Green spongy cushion Trentepohlia aurea Ulva californica Ulva fenestrata Sea lettuce Ulva intestinalis Sea hair, Sea lettuce, Gutweed, Grass kelp Ulva linza Ulva taeniata Urospora sp. Brown Algae or Ochrophyta Genus/Species Common Name Alaria marginata Ribbon kelp, Winged kelp Analipus japonicus Fir branch seaweed, Sea fir Coilodesme californica Dactylosiphon bullosus Desmarestia herbacea Desmarestia latifrons Egregia menziesii Feather boa Fucus distichus Bladderwrack, Rockweed Haplogloia andersonii Anderson's gooey brown Laminaria setchellii Southern stiff-stiped kelp Laminaria sinclairii Leathesia marina Sea cauliflower Melanosiphon intestinalis Twisted sea tubes Nereocystis luetkeana Bull kelp, Bullwhip kelp, Bladder wrack, Edible kelp, Ribbon kelp Pelvetiopsis limitata Petalonia fascia False kelp Petrospongium rugosum Phaeostrophion irregulare Sand-scoured false kelp Pterygophora californica Woody-stemmed kelp, Stalked kelp, Walking kelp Ralfsia sp. Silvetia compressa Rockweed Stephanocystis osmundacea Page 1 of 4 Red Algae or Rhodophyta Genus/Species Common Name Ahnfeltia fastigiata Bushy Ahnfelt's seaweed Ahnfeltiopsis linearis Anisocladella pacifica Bangia sp. Bossiella dichotoma Bossiella -

The Marine Life Information Network® for Britain and Ireland (Marlin)
The Marine Life Information Network® for Britain and Ireland (MarLIN) Description, temporal variation, sensitivity and monitoring of important marine biotopes in Wales. Volume 1. Background to biotope research. Report to Cyngor Cefn Gwlad Cymru / Countryside Council for Wales Contract no. FC 73-023-255G Dr Harvey Tyler-Walters, Charlotte Marshall, & Dr Keith Hiscock With contributions from: Georgina Budd, Jacqueline Hill, Will Rayment and Angus Jackson DRAFT / FINAL REPORT January 2005 Reference: Tyler-Walters, H., Marshall, C., Hiscock, K., Hill, J.M., Budd, G.C., Rayment, W.J. & Jackson, A., 2005. Description, temporal variation, sensitivity and monitoring of important marine biotopes in Wales. Report to Cyngor Cefn Gwlad Cymru / Countryside Council for Wales from the Marine Life Information Network (MarLIN). Marine Biological Association of the UK, Plymouth. [CCW Contract no. FC 73-023-255G] Description, sensitivity and monitoring of important Welsh biotopes Background 2 Description, sensitivity and monitoring of important Welsh biotopes Background The Marine Life Information Network® for Britain and Ireland (MarLIN) Description, temporal variation, sensitivity and monitoring of important marine biotopes in Wales. Contents Executive summary ............................................................................................................................................5 Crynodeb gweithredol ........................................................................................................................................6 -

Diversity of Plant Virus Movement Proteins: What Do They Have in Common?
processes Review Diversity of Plant Virus Movement Proteins: What Do They Have in Common? Yuri L. Dorokhov 1,2,* , Ekaterina V. Sheshukova 1, Tatiana E. Byalik 3 and Tatiana V. Komarova 1,2 1 Vavilov Institute of General Genetics Russian Academy of Sciences, 119991 Moscow, Russia; [email protected] (E.V.S.); [email protected] (T.V.K.) 2 Belozersky Institute of Physico-Chemical Biology, Lomonosov Moscow State University, 119991 Moscow, Russia 3 Department of Oncology, I.M. Sechenov First Moscow State Medical University, 119991 Moscow, Russia; [email protected] * Correspondence: [email protected] Received: 11 November 2020; Accepted: 24 November 2020; Published: 26 November 2020 Abstract: The modern view of the mechanism of intercellular movement of viruses is based largely on data from the study of the tobacco mosaic virus (TMV) 30-kDa movement protein (MP). The discovered properties and abilities of TMV MP, namely, (a) in vitro binding of single-stranded RNA in a non-sequence-specific manner, (b) participation in the intracellular trafficking of genomic RNA to the plasmodesmata (Pd), and (c) localization in Pd and enhancement of Pd permeability, have been used as a reference in the search and analysis of candidate proteins from other plant viruses. Nevertheless, although almost four decades have passed since the introduction of the term “movement protein” into scientific circulation, the mechanism underlying its function remains unclear. It is unclear why, despite the absence of homology, different MPs are able to functionally replace each other in trans-complementation tests. Here, we consider the complexity and contradictions of the approaches for assessment of the ability of plant viral proteins to perform their movement function. -
Chapter 4 – Cell Structure
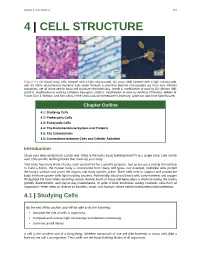
Chapter 4 | Cell Structure 107 4 | CELL STRUCTURE Figure 4.1 (a) Nasal sinus cells (viewed with a light microscope), (b) onion cells (viewed with a light microscope), and (c) Vibrio tasmaniensis bacterial cells (seen through a scanning electron microscope) are from very different organisms, yet all share certain basic cell structure characteristics. (credit a: modification of work by Ed Uthman, MD; credit b: modification of work by Umberto Salvagnin; credit c: modification of work by Anthony D'Onofrio, William H. Fowle, Eric J. Stewart, and Kim Lewis of the Lewis Lab at Northeastern University; scale-bar data from Matt Russell) Chapter Outline 4.1: Studying Cells 4.2: Prokaryotic Cells 4.3: Eukaryotic Cells 4.4: The Endomembrane System and Proteins 4.5: The Cytoskeleton 4.6: Connections between Cells and Cellular Activities Introduction Close your eyes and picture a brick wall. What is the wall's basic building block? It is a single brick. Like a brick wall, cells are the building blocks that make up your body. Your body has many kinds of cells, each specialized for a specific purpose. Just as we use a variety of materials to build a home, the human body is constructed from many cell types. For example, epithelial cells protect the body's surface and cover the organs and body cavities within. Bone cells help to support and protect the body. Immune system cells fight invading bacteria. Additionally, blood and blood cells carry nutrients and oxygen throughout the body while removing carbon dioxide. Each of these cell types plays a vital role during the body's growth, development, and day-to-day maintenance. -

Fucoid Algae As Model Organisms for Investigating Early Embryogenesis
Cah. Biol. Mar. (2001) 42 : 101-107 Fucoid algae as model organisms for investigating early embryogenesis Francois-Yves BOUGET1, Florence CORELLOU1 & Darryl L. KROPF2* 1 UMR 1931 CNRS-Goëmar, Station Biologique CNRS-INSU-Université Paris 6, Place Georges-Teissier, BP 74, F29682 Roscoff Cedex, France. 2 University of Utah, Department of Biology, 257 South 1400 East Salt Lake City, UT 84112-0840, USA E-mail: [email protected] * Author for correspondence Abstract: In the past few years, there have been exciting advances in our understanding of the mechanisms that control morphogenesis in fucoid embryos. In this article we review recent findings from our laboratories concerning 1) polarity establishment and expression in the zygote and 2) development of the zygote into a multicellular embryo. Résumé : Durant la dernière décennie, des avancées importantes ont été réalisées dans la compréhension des mécanismes qui contrôlent la morphogenèse des embryons de Fucacées. Dans cette revue, nous présentons les résultats récents obtenus dans nos laboratoires respectifs concernant 1) l’établissement de la polarité et son expression dans le zygote et 2) le déve- loppement du zygote en un embryon pluricellulaire. Keywords : Fucus, Pelvetia, embryogenesis, polarity. Introduction onto the substratum (rocks in the intertidal zone) where they attach tenaciously by a secreted adhesive (Vreeland et al., In addition to their importance as sources of natural 1993). Rapid adhesion is critical for survival because polymers and foods, many marine algae also provide zygotes that fail to attach are washed out to sea in the next excellent opportunities for investigating the mechanisms tidal cycle. As was first recognized over 100 years ago by that control development. -

Ascophyllum Nodosum) in Breiðafjörður, Iceland: Effects of Environmental Factors on Biomass and Plant Height
Rockweed (Ascophyllum nodosum) in Breiðafjörður, Iceland: Effects of environmental factors on biomass and plant height Lilja Gunnarsdóttir Faculty of Life and Environmental Sciences University of Iceland 2017 Rockweed (Ascophyllum nodosum) in Breiðafjörður, Iceland: Effects of environmental factors on biomass and plant height Lilja Gunnarsdóttir 60 ECTS thesis submitted in partial fulfillment of a Magister Scientiarum degree in Environment and Natural Resources MS Committee Mariana Lucia Tamayo Karl Gunnarsson Master’s Examiner Jörundur Svavarsson Faculty of Life and Environmental Science School of Engineering and Natural Sciences University of Iceland Reykjavik, December 2017 Rockweed (Ascophyllum nodosum) in Breiðafjörður, Iceland: Effects of environmental factors on biomass and plant height Rockweed in Breiðafjörður, Iceland 60 ECTS thesis submitted in partial fulfillment of a Magister Scientiarum degree in Environment and Natural Resources Copyright © 2017 Lilja Gunnarsdóttir All rights reserved Faculty of Life and Environmental Science School of Engineering and Natural Sciences University of Iceland Askja, Sturlugata 7 101, Reykjavik Iceland Telephone: 525 4000 Bibliographic information: Lilja Gunnarsdóttir, 2017, Rockweed (Ascophyllum nodosum) in Breiðafjörður, Iceland: Effects of environmental factors on biomass and plant height, Master’s thesis, Faculty of Life and Environmental Science, University of Iceland, pp. 48 Printing: Háskólaprent Reykjavik, Iceland, December 2017 Abstract During the Last Glacial Maximum (LGM) ice covered all rocky shores in eastern N-America while on the shores of Europe ice reached south of Ireland where rocky shores were found south of the glacier. After the LGM, rocky shores ecosystem development along European coasts was influenced mainly by movement of the littoral species in the wake of receding ice, while rocky shores of Iceland and NE-America were most likely colonized from N- Europe. -

Plasmodesmata and the Control of Symplastic Transport
Blackwell Science, LtdOxford, UKPCEPlant, Cell and Environment0016-8025Blackwell Publishing Ltd 2002 26 Original Article Plant, Cell and Environment (2003) 26, 103–124 Plasmodesmata and the control of symplastic transport A. G. ROBERTS & K. J. OPARKA Scottish Crop Research Institute, Invergowrie, Dundee, DD2 5DA, UK ‘There are holes in the sky other cells, some degree of intercellular connection is main- where the rain gets in, tained. This plasmodesmal continuum that potentially but they’re ever so small exists throughout the whole plant is termed the symplast that’s why rain’s so thin’ (Münch 1930). However, the symplast is not the open con- Spike Milligan (1968) tinuum that Münch originally hypothesized, but is divided into functional domains, each tightly regulated by different In 1879 Eduard Tangle discovered cytoplasmic connections forms of plasmodesmata (Erwee & Goodwin 1985; Ehlers between cells in the cotyledons of Strychnos nuxvomica, & Kollmann 2001). Plasmodesmata are now thought of as which he interpreted to be protoplasmic contacts. This led fluid, dynamic structures that can be modified both struc- him to hypothesize that ‘the protoplasmic bodies . are turally and functionally to cope with the requirements of united by thin strands passing through connecting ducts in specific cells and tissues. the walls, which put the cells into connection with each other and so unite them to an entity of higher order’ (Carr 1976). This challenged the then current view that cells func- THE STRUCTURE OF PLASMODESMATA tioned as autonomous units. It was after much research in Based on structure, two basic types of plasmodesmata have many other species and cell types that Strasburger, in 1901, been characterized; simple and branched. -

Marlin Marine Information Network Information on the Species and Habitats Around the Coasts and Sea of the British Isles
MarLIN Marine Information Network Information on the species and habitats around the coasts and sea of the British Isles Fucus distichus and Fucus spiralis f. nana on extremely exposed upper shore rock MarLIN – Marine Life Information Network Marine Evidence–based Sensitivity Assessment (MarESA) Review Frances Perry & Jacqueline Hill 2015-10-15 A report from: The Marine Life Information Network, Marine Biological Association of the United Kingdom. Please note. This MarESA report is a dated version of the online review. Please refer to the website for the most up-to-date version [https://www.marlin.ac.uk/habitats/detail/234]. All terms and the MarESA methodology are outlined on the website (https://www.marlin.ac.uk) This review can be cited as: Perry, F. & Hill, J.M., 2015. [Fucus distichus] and [Fucus spiralis f. nana] on extremely exposed upper shore rock. In Tyler-Walters H. and Hiscock K. (eds) Marine Life Information Network: Biology and Sensitivity Key Information Reviews, [on-line]. Plymouth: Marine Biological Association of the United Kingdom. DOI https://dx.doi.org/10.17031/marlinhab.234.1 The information (TEXT ONLY) provided by the Marine Life Information Network (MarLIN) is licensed under a Creative Commons Attribution-Non-Commercial-Share Alike 2.0 UK: England & Wales License. Note that images and other media featured on this page are each governed by their own terms and conditions and they may or may not be available for reuse. Permissions beyond the scope of this license are available here. Based on a work at www.marlin.ac.uk (page left blank) Date: 2015-10-15 Fucus distichus and Fucus spiralis f. -

Tobacco Mosaic Virus Movement Protein Associates with the Cytoskeleton in Tobacco Cells
The Plant Cell, Vol. 7, 2101-21 14, December 1995 O 1995 American Society of Plant Physiologists Tobacco Mosaic Virus Movement Protein Associates with the Cytoskeleton in Tobacco Cells 8. Gail McLean, John Zupan, and Patricia C. Zambryskil Department of Plant Biology, University of California-Berkeley, Berkeley, California 94720-3102 Tobacco mosaic virus movement protein P30 complexes with genomic viral RNA for transport through plasmodesmata, the plant intercellular connections. Although most research with P30 focuses on its targeting to and gating of plasmodes- mata, the mechanisms of P30 intracellularmovement to plasmodesmata have not been defined. To examine P30 intracellular localization, we used tobacco protoplasts, which lack plasmodesmata, for transfection with plasmids carrying P30 cod- ing sequences under a constitutive promoter and for infection with tobacco mosaic virus particles. In both systems, P30 appears as filaments that colocalize primarily with microtubules. To a lesser extent, P30 filaments colocalize with actin filaments, and in vitro experiments suggested that P30 can bind directly to actin and tubulin. This association of P30 with cytoskeletal elements may play a critical role in intracellular transport of the P30-vira1 RNA complex through the cytoplasm to and possibly through plasmodesmata. INTRODUCTION To establish a systemic infection, plant viruses must move from that the cytoskeleton acts as a trafficking system for intracel- the infection site to the rest of the plant. For many plant viruses, lular transport, translocating vesicles, organelles, protein, and a virus-encoded product, the movement protein, actively poten- even mRNA to specific cellular locations (Williamson, 1986; tiates viral cell-to-cell spread through plasmodesmata, the Vale, 1987; Dingwall, 1992; Singer, 1992; Wilhelm and Vale, cytoplasmic bridges that function as intercellular connections 1993; Bassell et al., 1994; Hesketh, 1994). -

Coalescence De L'écologie Du Paysage Littoral Et De La Technologie
Université du Québec INRS (Eau, Terre et Environnement) Coalescence de l’écologie du paysage littoral et de la technologie aéroportée du LiDAR ubiquiste THÈSE DE DOCTORAT Présentée pour l‘obtention du grade de Philosophiae Doctor (Ph.D.) en Sciences de la Terre Par Antoine Collin 19 mai 2009 Jury d‘évaluation Présidente du jury et Monique Bernier examinatrice interne Institut National de la Recherche Scientifique - Eau Terre et Environnement, Québec, Canada Examinateur interne Pierre Francus Institut National de la Recherche Scientifique - Eau Terre et Environnement, Québec, Canada Examinatrice externe Marie-Josée Fortin Université de Toronto, Ontario, Canada Examinateur externe Georges Stora Université de la Méditerranée, Marseille, France Directeur de recherche Bernard Long Institut National de la Recherche Scientifique – Eau, Terre et Environnement, Québec, Canada Co-directeur de recherche Philippe Archambault Institut des Sciences de la Mer, Université du Québec à Rimouski, Rimouski, Canada © Droits réservés de Antoine Collin, 2009 v Imprimée sur papier 100% recyclé « Nous croyons regarder la nature et c'est la nature qui nous regarde et nous imprègne. » Christian Charrière, Extrait de Le maître d'âme. vi vii Résumé La frange littorale englobe un éventail d‘écosystèmes dont les services écologiques atteignent 17.447 billions de dollars U.S., ce qui constitue la moitié de la somme totale des capitaux naturels des écosystèmes de la Terre. L‘accroissement démographique couplé aux bouleversements provoqués par le réchauffement climatique, génèrent inexorablement de fortes pressions sur les processus écologiques côtiers. L‘écologie du paysage, née de la rencontre de l‘écologie et de l‘aménagement du territoire, est susceptible d‘apporter les fondements scientifiques nécessaires à la gestion durable de ces écosystèmes littoraux. -

Organelle–Nucleus Cross-Talk Regulates Plant Intercellular
Organelle–nucleus cross-talk regulates plant PNAS PLUS intercellular communication via plasmodesmata Tessa M. Burch-Smitha, Jacob O. Brunkarda, Yoon Gi Choib, and Patricia C. Zambryskia,1 aDepartment of Plant and Microbial Biology and bFunctional Genomics Laboratory, University of California, Berkeley, CA 94720 Contributed by Patricia C. Zambryski, October 19, 2011 (sent for review June 20, 2011) We use Arabidopsis thaliana embryogenesis as a model system by either depositing or removing callose from the cell walls sur- for studying intercellular transport via plasmodesmata (PD). A rounding PD openings. PD also contain cytoskeleton-associated forward genetic screen for altered PD transport identified in- proteins, including actin (7) and myosin (8). Proteins with potential creased size exclusion limit (ise) 1 and ise2 mutants with increased roles in signal transduction are also PD constituents, such as a intercellular transport of fluorescent 10-kDa tracers. Both ise1 and calcium kinase (9), calreticulin (10), a kinase that phosphorylates ise2 exhibit increased formation of twinned and branched PD. viral movement proteins (11), and membrane receptor-like pro- ISE1 encodes a mitochondrial DEAD-box RNA helicase, whereas teins [PD-localized proteins (PDLPs)] (12). Remorin, a lipid-raft ISE2 encodes a DEVH-type RNA helicase. Here, we show that protein, was also recently found to localize to PD (13). Several ISE2 foci are localized to the chloroplast stroma. Surprisingly, additional potential membrane-bound PD-localized proteins have ise1 ise2 plastid development is defective in both and mutant recently been reported (14). embryos. In an effort to understand how RNA helicases that lo- We conducted a genetic screen of embryo-lethal mutants to calize to different organelles have similar impacts on plastid and identify genes critical to regulating PD function (15). -

Plasmodesmata in Higher Plants
2 PLASMODESMATA IN HIGHER PLANTS A.W. ROBARDS 1 Department of Developmental Biology, Research School of Biological Sciences, The Australian National University, Box 475, P.O., Canberra City, A.C.T. 2601, Australia 2.1. INTRODUCTION The first detailed description of "offene Communiaationen", or protoplasmic connecting threads is usually attributed to Tang1 (1879) although other workers subsequently contested his precedence. However, Tang1 was the first botanist to write at length on these structures and his article stimulated a spate of publications wbich described such connections between cells from all parts of the plant kingdom (for fuller details refer to Meeuse, 1941b; 1957, and Chapter 14). In 1901 Strasburger used the term "PZasmodesmen" to describe the protoplasmic connections and, despite numerous other suggestions (see Meeuse, 1957), the word has survived the test of time and is now almost universally accepted (English - plasmodesma - Gk. pZasma, form; desma, bond - P1u. plasmodesmata). Virtually all the early investigations involved treatment of cells to cause swelling of the wall so that plasmodesmata could be demonstrated by optical microscopy. This led to many critic isms and, as recently as 1964, Livingston reconsidered the "nature of pZasmodesmata in normaZ (Ziving) pZant tissue", so highlighting the problem that has been with us for over 80 years. The advent of elec tron microscopy has done much to expand knowledge about the structure and variability of plasmodesmata; it has been of rather less value in helping us to understand the true nature and physiological function of these connections. In this Chapter I shall collate some of the information relating to the distribution, structure and possible phys iological roles of plasmodesmata.