Changes in the Plasmodesma Structure and Permeability at the Bundle Sheath and Mesophyll Interface During the Maize C4 Leaf Development
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ATP-Citrate Lyase Has an Essential Role in Cytosolic Acetyl-Coa Production in Arabidopsis Beth Leann Fatland Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 2002 ATP-citrate lyase has an essential role in cytosolic acetyl-CoA production in Arabidopsis Beth LeAnn Fatland Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Molecular Biology Commons, and the Plant Sciences Commons Recommended Citation Fatland, Beth LeAnn, "ATP-citrate lyase has an essential role in cytosolic acetyl-CoA production in Arabidopsis " (2002). Retrospective Theses and Dissertations. 1218. https://lib.dr.iastate.edu/rtd/1218 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. ATP-citrate lyase has an essential role in cytosolic acetyl-CoA production in Arabidopsis by Beth LeAnn Fatland A dissertation submitted to the graduate faculty in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY Major: Plant Physiology Program of Study Committee: Eve Syrkin Wurtele (Major Professor) James Colbert Harry Homer Basil Nikolau Martin Spalding Iowa State University Ames, Iowa 2002 UMI Number: 3158393 INFORMATION TO USERS The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleed-through, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. -

Diversity of Plant Virus Movement Proteins: What Do They Have in Common?
processes Review Diversity of Plant Virus Movement Proteins: What Do They Have in Common? Yuri L. Dorokhov 1,2,* , Ekaterina V. Sheshukova 1, Tatiana E. Byalik 3 and Tatiana V. Komarova 1,2 1 Vavilov Institute of General Genetics Russian Academy of Sciences, 119991 Moscow, Russia; [email protected] (E.V.S.); [email protected] (T.V.K.) 2 Belozersky Institute of Physico-Chemical Biology, Lomonosov Moscow State University, 119991 Moscow, Russia 3 Department of Oncology, I.M. Sechenov First Moscow State Medical University, 119991 Moscow, Russia; [email protected] * Correspondence: [email protected] Received: 11 November 2020; Accepted: 24 November 2020; Published: 26 November 2020 Abstract: The modern view of the mechanism of intercellular movement of viruses is based largely on data from the study of the tobacco mosaic virus (TMV) 30-kDa movement protein (MP). The discovered properties and abilities of TMV MP, namely, (a) in vitro binding of single-stranded RNA in a non-sequence-specific manner, (b) participation in the intracellular trafficking of genomic RNA to the plasmodesmata (Pd), and (c) localization in Pd and enhancement of Pd permeability, have been used as a reference in the search and analysis of candidate proteins from other plant viruses. Nevertheless, although almost four decades have passed since the introduction of the term “movement protein” into scientific circulation, the mechanism underlying its function remains unclear. It is unclear why, despite the absence of homology, different MPs are able to functionally replace each other in trans-complementation tests. Here, we consider the complexity and contradictions of the approaches for assessment of the ability of plant viral proteins to perform their movement function. -
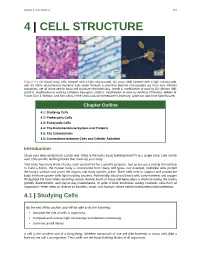
Chapter 4 – Cell Structure
Chapter 4 | Cell Structure 107 4 | CELL STRUCTURE Figure 4.1 (a) Nasal sinus cells (viewed with a light microscope), (b) onion cells (viewed with a light microscope), and (c) Vibrio tasmaniensis bacterial cells (seen through a scanning electron microscope) are from very different organisms, yet all share certain basic cell structure characteristics. (credit a: modification of work by Ed Uthman, MD; credit b: modification of work by Umberto Salvagnin; credit c: modification of work by Anthony D'Onofrio, William H. Fowle, Eric J. Stewart, and Kim Lewis of the Lewis Lab at Northeastern University; scale-bar data from Matt Russell) Chapter Outline 4.1: Studying Cells 4.2: Prokaryotic Cells 4.3: Eukaryotic Cells 4.4: The Endomembrane System and Proteins 4.5: The Cytoskeleton 4.6: Connections between Cells and Cellular Activities Introduction Close your eyes and picture a brick wall. What is the wall's basic building block? It is a single brick. Like a brick wall, cells are the building blocks that make up your body. Your body has many kinds of cells, each specialized for a specific purpose. Just as we use a variety of materials to build a home, the human body is constructed from many cell types. For example, epithelial cells protect the body's surface and cover the organs and body cavities within. Bone cells help to support and protect the body. Immune system cells fight invading bacteria. Additionally, blood and blood cells carry nutrients and oxygen throughout the body while removing carbon dioxide. Each of these cell types plays a vital role during the body's growth, development, and day-to-day maintenance. -

Development and Function of Plasmodesmata in Zygotes of Fucus Distichus
Botanica Marina 2015; 58(3): 229–238 Chikako Nagasato*, Makoto Terauchi, Atsuko Tanaka and Taizo Motomura Development and function of plasmodesmata in zygotes of Fucus distichus Abstract: Brown algae have plasmodesmata, tiny tubu- Introduction lar cytoplasmic channels connecting adjacent cells. The lumen of plasmodesmata is 10–20 nm wide, and it takes a Multicellular organisms such as animals, fungi, land simple form, without a desmotubule (the inner membrane plants, and brown algae have specific cellular connections. structure consisting of endoplasmic reticulum in the plas- These structures connect the cytoplasm of adjacent cells modesmata of green plants). In this study, we analyzed and provide a route for cell-cell communication. Green the ultrastructure and distribution of plasmodesmata plants, including land plants and certain species of green during development of Fucus distichus zygotes. The first algae (reviewed in Robards and Lucas 1990, Raven 1997), cytokinesis of zygotes in brown algae is not accompanied and brown algae (Bisalputra 1966) possess plasmodes- by plasmodesmata formation. As the germlings develop, mata. These are cytoplasmic canals that pass through the plasmodesmata are found in all septal cell walls, includ- septal cell wall and connect adjacent cells. Plasmodes- ing the first cell division plane. Plasmodesmata are formed mata in green plants and brown algae share similar char- de novo on the existing cell wall. Pit fields, which are clus- acteristics; however, plasmodesmata in brown algae lack ters of plasmodesmata, were observed in germlings with a desmotubule, a tubular strand of connecting endoplas- differentiated cell layers. Apart from the normal plas- mic reticulum (ER) that penetrates the plasmodesmata modesmata, these pit fields had branched plasmodes- of land plants (Terauchi et al. -

On the Determínation of Suberin and Other Structural Components in Cork from Quercus Suber L
Recebido em 14 de Dezembro de 1987 On the determínation of suberin and other structural components in cork from Quercus suber L. by A. VELEZ MARQUES Dep. Quím. Org., Instituto Superior de Engenharia de Lisboa and HELENA PEREIRA Dep. Eng. Florestal, Instituto Superior de Agronomia RESUMO Estudou-se a despolimerização da suberina na cortiça de Quercus suber L. em relação à influência da concentração de reagente e tempo de reacção, utilizando uma transesterificação com metóxido de sódio em metanol. A de terminação da lenhina e dos polisacáridos foi feita em cortiça dessuberinizada. A quantidade de suberina aumenta com a concentração de metóxido, ao contrário do que se passa para os outros componentes; o mesmo se observa com o aumento do tempo da reacção. A composição monomérica dos polisacáridos mantém-se sensivelmente constante para todas as condições de metanólise. Propõe-se um método de despolimerização de suberina utilizando metóxido de sódio a 3% em metanol, em refluxo durante 3h, para uma amostra de l,5g de cortiça livre de extractivos, de modo a maximizar a despolimerização da suberina mas com menores efeitos nas determinações subsequentes dos outros componentes estruturais. 322 ANAIS DO INSTITUTO SUPERIOR DE AGRONOMIA SYNOPSIS The depolymerization of suberin in cork from Quercus suber L. was stu- died in relation to the effect of reagent concentration and reaction time, using a transesterification witli sodium methoxide in methanol. Lignin and carbohy- drates were determined in the desuberinised cork samples. The amount of suberin increases with the concentration of methoxide con- trarily to the other componentes; the same effect is observed with an increase of reaction time. -

Plasmodesmata and the Control of Symplastic Transport
Blackwell Science, LtdOxford, UKPCEPlant, Cell and Environment0016-8025Blackwell Publishing Ltd 2002 26 Original Article Plant, Cell and Environment (2003) 26, 103–124 Plasmodesmata and the control of symplastic transport A. G. ROBERTS & K. J. OPARKA Scottish Crop Research Institute, Invergowrie, Dundee, DD2 5DA, UK ‘There are holes in the sky other cells, some degree of intercellular connection is main- where the rain gets in, tained. This plasmodesmal continuum that potentially but they’re ever so small exists throughout the whole plant is termed the symplast that’s why rain’s so thin’ (Münch 1930). However, the symplast is not the open con- Spike Milligan (1968) tinuum that Münch originally hypothesized, but is divided into functional domains, each tightly regulated by different In 1879 Eduard Tangle discovered cytoplasmic connections forms of plasmodesmata (Erwee & Goodwin 1985; Ehlers between cells in the cotyledons of Strychnos nuxvomica, & Kollmann 2001). Plasmodesmata are now thought of as which he interpreted to be protoplasmic contacts. This led fluid, dynamic structures that can be modified both struc- him to hypothesize that ‘the protoplasmic bodies . are turally and functionally to cope with the requirements of united by thin strands passing through connecting ducts in specific cells and tissues. the walls, which put the cells into connection with each other and so unite them to an entity of higher order’ (Carr 1976). This challenged the then current view that cells func- THE STRUCTURE OF PLASMODESMATA tioned as autonomous units. It was after much research in Based on structure, two basic types of plasmodesmata have many other species and cell types that Strasburger, in 1901, been characterized; simple and branched. -

The Age of Coumarins in Plant–Microbe Interactions Pca Issue Special Ioannis A
The Age of Coumarins in Plant–Microbe Interactions Special Issue Ioannis A. Stringlis *, Ronnie de Jonge and Corne´ M. J. Pieterse Plant-Microbe Interactions, Department of Biology, Science4Life, Utrecht University, Padualaan 8, Utrecht, 3584 CH, The Netherlands *Corresponding author: E-mail, [email protected]; Fax,+31 30 253 2837. (Received February 9, 2019; Accepted April 23, 2019) Coumarins are a family of plant-derived secondary metab- For example, the cell wall-fortifying compounds lignin, cutin olites that are produced via the phenylpropanoid pathway. and suberin form structural barriers that inhibit pathogen in- In the past decade, coumarins have emerged as iron-mobi- vasion (Doblas et al. 2017). Other phenylpropanoid derivatives – Review lizing compounds that are secreted by plant roots and aid in such as flavonoids, anthocyanins and tannins participate in iron uptake from iron-deprived soils. Members of the cou- other aspects of environmental stress adaptation, or in plant marin family are found in many plant species. Besides their growth and physiology (Vogt 2010). More specifically, flavon- role in iron uptake, coumarins have been extensively studied oids emerged as important mediators of the chemical commu- for their potential to fight infections in both plants and nication between leguminous plants and beneficial nitrogen- animals. Coumarin activities range from antimicrobial and fixing rhizobia. In this mutualistic interaction, root-secreted fla- antiviral to anticoagulant and anticancer. In recent years, vonoids act as chemoattractants for rhizobia and activate genes studies in the model plant species tobacco and required for nodulation, which established the initial paradigm Arabidopsis have significantly increased our understanding for the role phenylpropanoid-derived metabolites in beneficial of coumarin biosynthesis, accumulation, secretion, chemical plant–microbe interactions (Fisher and Long 1992, Phillips modification and their modes of action against plant patho- 1992). -

Tobacco Mosaic Virus Movement Protein Associates with the Cytoskeleton in Tobacco Cells
The Plant Cell, Vol. 7, 2101-21 14, December 1995 O 1995 American Society of Plant Physiologists Tobacco Mosaic Virus Movement Protein Associates with the Cytoskeleton in Tobacco Cells 8. Gail McLean, John Zupan, and Patricia C. Zambryskil Department of Plant Biology, University of California-Berkeley, Berkeley, California 94720-3102 Tobacco mosaic virus movement protein P30 complexes with genomic viral RNA for transport through plasmodesmata, the plant intercellular connections. Although most research with P30 focuses on its targeting to and gating of plasmodes- mata, the mechanisms of P30 intracellularmovement to plasmodesmata have not been defined. To examine P30 intracellular localization, we used tobacco protoplasts, which lack plasmodesmata, for transfection with plasmids carrying P30 cod- ing sequences under a constitutive promoter and for infection with tobacco mosaic virus particles. In both systems, P30 appears as filaments that colocalize primarily with microtubules. To a lesser extent, P30 filaments colocalize with actin filaments, and in vitro experiments suggested that P30 can bind directly to actin and tubulin. This association of P30 with cytoskeletal elements may play a critical role in intracellular transport of the P30-vira1 RNA complex through the cytoplasm to and possibly through plasmodesmata. INTRODUCTION To establish a systemic infection, plant viruses must move from that the cytoskeleton acts as a trafficking system for intracel- the infection site to the rest of the plant. For many plant viruses, lular transport, translocating vesicles, organelles, protein, and a virus-encoded product, the movement protein, actively poten- even mRNA to specific cellular locations (Williamson, 1986; tiates viral cell-to-cell spread through plasmodesmata, the Vale, 1987; Dingwall, 1992; Singer, 1992; Wilhelm and Vale, cytoplasmic bridges that function as intercellular connections 1993; Bassell et al., 1994; Hesketh, 1994). -

Global Transcriptome Analysis and Characterization of Dryopteris Fragrans
Lu et al. BMC Genomics (2018) 19:471 https://doi.org/10.1186/s12864-018-4843-2 RESEARCHARTICLE Open Access Global transcriptome analysis and characterization of Dryopteris fragrans (L.) Schott sporangium in different developmental stages Zhen Lu1, Qingyang Huang1,3, Tong Zhang1, Baozhong Hu2* and Ying Chang1* Abstract Background: Dryopteris fragrans (D. fragrans) is a potential medicinal fern distributed in volcanic magmatic rock areas under tough environmental condition. Sporangia are important organs for fern reproduction. This study was designed to characterize the transcriptome characteristics of the wild D. fragrans sporangia in three stages (stage A, B, and C) with the aim of uncovering its molecular mechanism of growth and development. Results: Using a HiSeq 4000, 79.81 Gb clean data (each sample is at least 7.95 GB) were obtained from nine samples, with three being supplied from each period, and assembled into 94,705 Unigenes, among which 44,006 Unigenes were annotated against public protein databases (NR, Swiss-Prot, KEGG, COG, KOG, GO, eggNOG and Pfam). Furthermore, we observed 7126 differentially expressed genes (DEG) (Fold Change > 4, FDR < 0.001), 349,885 SNP loci, and 10,584 SSRs. DEGs involved in DNA replication and homologous recombination were strongly expressed in stage A, and several DEGs involved in cutin, suberin and wax biosynthesis had undergone dramatic changes during development, which was consistent with morphological observations. DEGs responsible for secondary metabolism and plant hormone signal transduction changed clearly in the last two stages. DEGs homologous to those known genes associated with the development of reproductive organs of flowering plants have also been validated and discussed, such as AGL61, AGL62, ONAC010. -

Organelle–Nucleus Cross-Talk Regulates Plant Intercellular
Organelle–nucleus cross-talk regulates plant PNAS PLUS intercellular communication via plasmodesmata Tessa M. Burch-Smitha, Jacob O. Brunkarda, Yoon Gi Choib, and Patricia C. Zambryskia,1 aDepartment of Plant and Microbial Biology and bFunctional Genomics Laboratory, University of California, Berkeley, CA 94720 Contributed by Patricia C. Zambryski, October 19, 2011 (sent for review June 20, 2011) We use Arabidopsis thaliana embryogenesis as a model system by either depositing or removing callose from the cell walls sur- for studying intercellular transport via plasmodesmata (PD). A rounding PD openings. PD also contain cytoskeleton-associated forward genetic screen for altered PD transport identified in- proteins, including actin (7) and myosin (8). Proteins with potential creased size exclusion limit (ise) 1 and ise2 mutants with increased roles in signal transduction are also PD constituents, such as a intercellular transport of fluorescent 10-kDa tracers. Both ise1 and calcium kinase (9), calreticulin (10), a kinase that phosphorylates ise2 exhibit increased formation of twinned and branched PD. viral movement proteins (11), and membrane receptor-like pro- ISE1 encodes a mitochondrial DEAD-box RNA helicase, whereas teins [PD-localized proteins (PDLPs)] (12). Remorin, a lipid-raft ISE2 encodes a DEVH-type RNA helicase. Here, we show that protein, was also recently found to localize to PD (13). Several ISE2 foci are localized to the chloroplast stroma. Surprisingly, additional potential membrane-bound PD-localized proteins have ise1 ise2 plastid development is defective in both and mutant recently been reported (14). embryos. In an effort to understand how RNA helicases that lo- We conducted a genetic screen of embryo-lethal mutants to calize to different organelles have similar impacts on plastid and identify genes critical to regulating PD function (15). -

Plasmodesmata in Higher Plants
2 PLASMODESMATA IN HIGHER PLANTS A.W. ROBARDS 1 Department of Developmental Biology, Research School of Biological Sciences, The Australian National University, Box 475, P.O., Canberra City, A.C.T. 2601, Australia 2.1. INTRODUCTION The first detailed description of "offene Communiaationen", or protoplasmic connecting threads is usually attributed to Tang1 (1879) although other workers subsequently contested his precedence. However, Tang1 was the first botanist to write at length on these structures and his article stimulated a spate of publications wbich described such connections between cells from all parts of the plant kingdom (for fuller details refer to Meeuse, 1941b; 1957, and Chapter 14). In 1901 Strasburger used the term "PZasmodesmen" to describe the protoplasmic connections and, despite numerous other suggestions (see Meeuse, 1957), the word has survived the test of time and is now almost universally accepted (English - plasmodesma - Gk. pZasma, form; desma, bond - P1u. plasmodesmata). Virtually all the early investigations involved treatment of cells to cause swelling of the wall so that plasmodesmata could be demonstrated by optical microscopy. This led to many critic isms and, as recently as 1964, Livingston reconsidered the "nature of pZasmodesmata in normaZ (Ziving) pZant tissue", so highlighting the problem that has been with us for over 80 years. The advent of elec tron microscopy has done much to expand knowledge about the structure and variability of plasmodesmata; it has been of rather less value in helping us to understand the true nature and physiological function of these connections. In this Chapter I shall collate some of the information relating to the distribution, structure and possible phys iological roles of plasmodesmata. -

A 13C NMR Study Using Isotopically Labeled Precursors
3298 J. Agric. Food Chem. 2000, 48, 3298−3304 Biosynthesis, Molecular Structure, and Domain Architecture of Potato Suberin: A 13C NMR Study Using Isotopically Labeled Precursors Bin Yan and Ruth E. Stark* Department of Chemistry, Graduate School and College of Staten Island of the City University of New York, 2800 Victory Boulevard, Staten Island, New York 10314 Although suberin in potato wound periderm is known to be a polyester containing long-chain fatty acids and phenolics embedded within the cell wall, many aspects of its molecular structure and polymer-polymer connectivities remain elusive. The present work combines biosynthetic incorpora- tion of site-specifically 13C-enriched acetates and phenylalanines with one- and two-dimensional solid-state 13C NMR spectroscopic methods to monitor the developing suberin polymer. Exogenous acetate is found to be incorporated preferentially at the carboxyl end of the aliphatic carbon chains, suggesting addition during the later elongation steps of fatty acid synthesis. Carboxyl-labeled phenylalanine precursors provide evidence for the concurrent development of phenolic esters and of monolignols typical of lignin. Experiments with ring-labeled phenylalanine precursors demonstrate a predominance of sinapyl and guaiacyl structures among suberin’s phenolic moieties. Finally, the analysis of spin-exchange (solid-state NOESY) NMR experiments in ring-labeled suberin indicates distances of no more than 0.5 nm between pairs of phenolic and oxymethine carbons, which are attributed to the aromatic-aliphatic polyester and the cell wall polysaccharide matrix, respectively. These results offer direct and detailed molecular information regarding the insoluble intermediates of suberin biosynthesis, indicate probable covalent linkages between moieties of its polyester and polysaccharide domains, and yield a clearer overall picture of this agriculturally important protective material.