Diversity of Plant Virus Movement Proteins: What Do They Have in Common?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

GENES and SEQUENCES INVOLVED in the REPLICATION of COWPEA MOSAIC VIRUS Rnas
GENES AND SEQUENCES INVOLVED INTH E REPLICATION OF COWPEA MOSAIC VIRUS RNA s CENTRALE LANDBO UW CATALO GU S 0000 0330 0924 Promotoren: Dr. A. van Kammen,hoogleraa r ind e moleculaire biologie Dr. R.W. Goldbach,hoogleraa r ind e virologie Rik Eggen GENES AND SEQUENCES INVOLVED IN THE REPLICATION OF COWPEA MOSAIC VIRUS RNAs Proefschrift ter verkrijging vand egraa d van doctor in de landbouwwetenschappen, op gezagva nd e rector magnificus, dr. H.C. van der Plas, in het openbaar te verdedigen op woensdag 3me i 1989 des namiddags te vier uur in de aula vand e Landbouwuniversiteit te Wageningen \m x<\\*\o'i> Thiswor k wassupporte d byth e Netherlands Foundation for Chemical Research (SON) with financial aidfro m the Netherlands Organization for Scientific Research (NWO). The printing ofthi sthesi s wasfinanciall y supported byAmersha m and Gibco BRL. 1989 Druk: SSN Nijmegen mo%vD\,n^ STELLINGEN Dehomologi e tussen cowpeamosai c virus (CPMV)e n poliovirus geldt voor structurele kenmerken maar niet voor overeenkomende mechanismen vand e virusvermenigvuldiging. Goldbach (1987)Microbiologica l Sciences 4,197-202 . Wellink (1987)Proefschrif cL UWageningen . Ditproefschrift . De door CPMV gecodeerde 87e n 110kilodalto n eiwitten kunnen de synthese van een complementaire RNAkete n niet initiëren. Dit proefschrift. Voor het verkrijgen van stabiele mutaties in specifieke gebieden van het CPMV genoom zal randommutagenes e van een DNA cassette een kansrijkere manier zijn dan plaats gerichte mutagenese. De experimenten van Jang et ale n Pelletier en Sonenberg tonen onomstotelijk aan dat picornaviraal RNA zich niet conformeert aan het ribosoom scanningmode lda t isopgestel d voor eukaryotische messenger RNAs. -

Effectiveness of Seed Treatments in Reducing TMV Infection Tanner A
Effectiveness of Seed Treatments in Reducing TMV Infection Tanner A. Robison Mentor: Claudia Nischwitz Department of Biology, Utah State University, Logan, Utah, USA Abstract Germination Rate Tobacco mosaic virus (TMV), named for the plant it was first discovered in, 1 can infect hundreds of different species. It has no known invertebrate 0.9 vectors, but TMV is spread mechanically through contact with other plants, 0.8 clothes, tools, contaminated soil and seedborne. While TMV is known to be 0.7 exceptionally stable, it has been reported that treatment of tools with 0.6 powdered milk can significantly reduce the infection rate of the virus. While 0.5 there is a seed treatment available for conventional agriculture, there is no 0.4 seed treatment available for organic production. Organic growers, who grow 0.3 heirloom tomatoes experience the highest rates of TMV infection. Since 0.2 powdered milk has been reported as an effective means of treatment in 0.1 tools, the purpose of this research is to determine whether it can be an 0 Almond Soy Rinsed Unrinsed Control effective seed treatment. To test this, tomato seeds were treated with powdered milk, soy milk, almond milk and water. A grow out test was then Results/Discussion conducted in a greenhouse and plants were tested for infection using In the grow out trial little effect was seen. The infection rate for Polymerase Chain Reaction and antibody based ELISA testing. In the first grow powdered milk, almond milk, soy milk and control treatments out trial treatment little effect was seen, with infection rate for the powdered were 61%, 34%, 32% and 47%, respectively. -

Induction of Plant Resistance Against Tobacco Mosaic Virus Using the Biocontrol Agent Streptomyces Cellulosae Isolate Actino 48
agronomy Article Induction of Plant Resistance against Tobacco Mosaic Virus Using the Biocontrol Agent Streptomyces cellulosae Isolate Actino 48 Gaber Attia Abo-Zaid 1 , Saleh Mohamed Matar 1,2 and Ahmed Abdelkhalek 3,* 1 Bioprocess Development Department, Genetic Engineering and Biotechnology Research Institute (GEBRI), City of Scientific Research and Technological Applications (SRTA-City), New Borg El-Arab City, Alexandria 21934, Egypt; [email protected] (G.A.A.-Z.); [email protected] (S.M.M.) 2 Chemical Engineering Department, Faculty of Engineering, Jazan University, Jazan 45142, Saudi Arabia 3 Plant Protection and Biomolecular Diagnosis Department, ALCRI, City of Scientific Research and Technological Applications, New Borg El Arab city, Alexandria 21934, Egypt * Correspondence: [email protected] Received: 8 September 2020; Accepted: 19 October 2020; Published: 22 October 2020 Abstract: Viral plant diseases represent a serious problem in agricultural production, causing large shortages in the production of food crops. Eco-friendly approaches are used in controlling viral plant infections, such as biocontrol agents. In the current study, Streptomyces cellulosae isolate Actino 48 is tested as a biocontrol agent for the management of tobacco mosaic virus (TMV) and inducing tomato plant systemic resistance under greenhouse conditions. Foliar application of a cell pellet suspension of Actino 48 (2 107 cfu. mL 1) is performed at 48 h before inoculation with TMV. Peroxidase activity, × − chitinase activity, protein content, and the total phenolic compounds are measured in tomato leaves at 21 dpi. On the other hand, the TMV accumulation level and the transcriptional changes of five tomato defense-related genes (PAL, PR-1, CHS, PR-3, and PR-2) are studied. -

The Cell Biology of Tobacco Mosaic Virus Replication and Movement
REVIEW ARTICLE published: 11 February 2013 doi: 10.3389/fpls.2013.00012 The cell biology ofTobacco mosaic virus replication and movement Chengke Liu and Richard S. Nelson* Plant Biology Division, The Samuel Roberts Noble Foundation, Inc., Ardmore, OK, USA Edited by: Successful systemic infection of a plant by Tobacco mosaic virus (TMV) requires three Jean-François Laliberté, Institut processes that repeat over time: initial establishment and accumulation in invaded cells, National de la Recherche Scientifique, Canada intercellular movement, and systemic transport. Accumulation and intercellular movement of TMV necessarily involves intracellular transport by complexes containing virus and Reviewed by: Xueping Zhou, Zhejiang University, host proteins and virus RNA during a dynamic process that can be visualized. Multiple China membranes appear to assist TMV accumulation, while membranes, microfilaments and Yule Liu, Tsinghua University, China microtubules appear to assist TMV movement. Here we review cell biological studies *Correspondence: that describe TMV-membrane, -cytoskeleton, and -other host protein interactions which Richard S. Nelson, Plant Biology influence virus accumulation and movement in leaves and callus tissue. The importance Division, The Samuel Roberts Noble Foundation, Inc., 2510 Sam Noble of understanding the developmental phase of the infection in relationship to the observed Parkway, Ardmore, OK 73401, USA. virus-membrane or -host protein interaction is emphasized. Utilizing the latest observations e-mail: [email protected] of TMV-membrane and -host protein interactions within our evolving understanding of the infection ontogeny, a model forTMV accumulation and intracellular spread in a cell biological context is provided. Keywords: membrane transport, microfilaments, microtubules, plant virus, vesicle trafficking, tobamovirus INTRODUCTION of the observed outcome on the mechanism of virus movement is Viruses, as obligate organisms, utilize host factors to accumu- noted. -

Comparison of Plant‐Adapted Rhabdovirus Protein Localization and Interactions
University of Kentucky UKnowledge University of Kentucky Doctoral Dissertations Graduate School 2011 COMPARISON OF PLANT‐ADAPTED RHABDOVIRUS PROTEIN LOCALIZATION AND INTERACTIONS Kathleen Marie Martin University of Kentucky, [email protected] Right click to open a feedback form in a new tab to let us know how this document benefits ou.y Recommended Citation Martin, Kathleen Marie, "COMPARISON OF PLANT‐ADAPTED RHABDOVIRUS PROTEIN LOCALIZATION AND INTERACTIONS" (2011). University of Kentucky Doctoral Dissertations. 172. https://uknowledge.uky.edu/gradschool_diss/172 This Dissertation is brought to you for free and open access by the Graduate School at UKnowledge. It has been accepted for inclusion in University of Kentucky Doctoral Dissertations by an authorized administrator of UKnowledge. For more information, please contact [email protected]. ABSTRACT OF DISSERTATION Kathleen Marie Martin The Graduate School University of Kentucky 2011 COMPARISON OF PLANT‐ADAPTED RHABDOVIRUS PROTEIN LOCALIZATION AND INTERACTIONS ABSTRACT OF DISSERTATION A dissertation submitted in partial fulfillment of the requirements for the Degree of Doctor of Philosophy in the College of Agriculture at the University of Kentucky By Kathleen Marie Martin Lexington, Kentucky Director: Dr. Michael M Goodin, Associate Professor of Plant Pathology Lexington, Kentucky 2011 Copyright © Kathleen Marie Martin 2011 ABSTRACT OF DISSERTATION COMPARISON OF PLANT‐ADAPTED RHABDOVIRUS PROTEIN LOCALIZATION AND INTERACTIONS Sonchus yellow net virus (SYNV), Potato yellow dwarf virus (PYDV) and Lettuce Necrotic yellows virus (LNYV) are members of the Rhabdoviridae family that infect plants. SYNV and PYDV are Nucleorhabdoviruses that replicate in the nuclei of infected cells and LNYV is a Cytorhabdovirus that replicates in the cytoplasm. LNYV and SYNV share a similar genome organization with a gene order of Nucleoprotein (N), Phosphoprotein (P), putative movement protein (Mv), Matrix protein (M), Glycoprotein (G) and Polymerase protein (L). -

Tomato, Tobacco Mosaic Virus
Problem: Tobacco Mosaic Virus of Tomato Host Plants: Tomato, pepper, eggplant, tobacco, spinach, petunia, marigold. Description: Several virus diseases to tomato occur in Kansas, although they generally are not as prevalent as the wilt and foliar diseases. Three of the more common virus diseases are tobacco mosaic, cucumber mosaic, and spotted wilt. The tobacco mosaic virus can attack a wide range of plants, including tomato, pepper, eggplant, tobacco, spinach, petunia and marigold. On tomato, virus infection causes light and dark green mottled areas on the leaves. The dark green areas tend to be somewhat thicker than the lighter portions of the leaf. The leaf mottling is seen more easily if the affected plant surface is partially shaded. Stunting of young plants is common and often is accompanied by a distortion and fern-like appearance of the leaves. Older leaves curl downward and may be slightly distorted. Certain strains of the virus can cause a mottling, streaking and necrosis of the fruits. Infected plants are not killed, but they produce poor quality fruit and low yields. Tobacco mosaic, is incited by a virus. The tobacco mosaic virus is very stable and can persist in contaminated soil, in infected tomato debris, on or in the seed coat, and in manufactured tobacco products. The virus is transmitted readily from plant to plant by mechanical means. This may simply involve picking up the virus while working with infected plant material, then inoculating healthy plants by rubbing or brushing against them with contaminated tools, clothing, or hands. Aphids are not vectors of the virus, although certain chewing insects may transmit the pathogen. -
Chapter 4 – Cell Structure
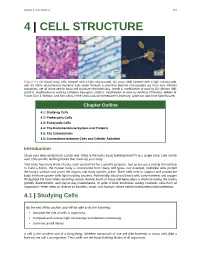
Chapter 4 | Cell Structure 107 4 | CELL STRUCTURE Figure 4.1 (a) Nasal sinus cells (viewed with a light microscope), (b) onion cells (viewed with a light microscope), and (c) Vibrio tasmaniensis bacterial cells (seen through a scanning electron microscope) are from very different organisms, yet all share certain basic cell structure characteristics. (credit a: modification of work by Ed Uthman, MD; credit b: modification of work by Umberto Salvagnin; credit c: modification of work by Anthony D'Onofrio, William H. Fowle, Eric J. Stewart, and Kim Lewis of the Lewis Lab at Northeastern University; scale-bar data from Matt Russell) Chapter Outline 4.1: Studying Cells 4.2: Prokaryotic Cells 4.3: Eukaryotic Cells 4.4: The Endomembrane System and Proteins 4.5: The Cytoskeleton 4.6: Connections between Cells and Cellular Activities Introduction Close your eyes and picture a brick wall. What is the wall's basic building block? It is a single brick. Like a brick wall, cells are the building blocks that make up your body. Your body has many kinds of cells, each specialized for a specific purpose. Just as we use a variety of materials to build a home, the human body is constructed from many cell types. For example, epithelial cells protect the body's surface and cover the organs and body cavities within. Bone cells help to support and protect the body. Immune system cells fight invading bacteria. Additionally, blood and blood cells carry nutrients and oxygen throughout the body while removing carbon dioxide. Each of these cell types plays a vital role during the body's growth, development, and day-to-day maintenance. -

Development and Function of Plasmodesmata in Zygotes of Fucus Distichus
Botanica Marina 2015; 58(3): 229–238 Chikako Nagasato*, Makoto Terauchi, Atsuko Tanaka and Taizo Motomura Development and function of plasmodesmata in zygotes of Fucus distichus Abstract: Brown algae have plasmodesmata, tiny tubu- Introduction lar cytoplasmic channels connecting adjacent cells. The lumen of plasmodesmata is 10–20 nm wide, and it takes a Multicellular organisms such as animals, fungi, land simple form, without a desmotubule (the inner membrane plants, and brown algae have specific cellular connections. structure consisting of endoplasmic reticulum in the plas- These structures connect the cytoplasm of adjacent cells modesmata of green plants). In this study, we analyzed and provide a route for cell-cell communication. Green the ultrastructure and distribution of plasmodesmata plants, including land plants and certain species of green during development of Fucus distichus zygotes. The first algae (reviewed in Robards and Lucas 1990, Raven 1997), cytokinesis of zygotes in brown algae is not accompanied and brown algae (Bisalputra 1966) possess plasmodes- by plasmodesmata formation. As the germlings develop, mata. These are cytoplasmic canals that pass through the plasmodesmata are found in all septal cell walls, includ- septal cell wall and connect adjacent cells. Plasmodes- ing the first cell division plane. Plasmodesmata are formed mata in green plants and brown algae share similar char- de novo on the existing cell wall. Pit fields, which are clus- acteristics; however, plasmodesmata in brown algae lack ters of plasmodesmata, were observed in germlings with a desmotubule, a tubular strand of connecting endoplas- differentiated cell layers. Apart from the normal plas- mic reticulum (ER) that penetrates the plasmodesmata modesmata, these pit fields had branched plasmodes- of land plants (Terauchi et al. -

Corona and Other Virus: Their Useful and Harmful Aspects
Current Trends on Biotechnology & Microbiology DOI: ISSN: 2641-6875 10.32474/CTBM.2020.02.000129Review Article Corona and Other Virus: Their Useful and Harmful Aspects Birhanu Gizaw* Microbial Biodiversity Directorate, Ethiopian Biodiversity Institute, Ethiopia *Corresponding author: Birhanu Gizaw, Microbial Biodiversity Directorate, Ethiopian Biodiversity Institute, P.O. Box 30726, Addis Ababa, Ethiopia Received: June 15, 2020 Published: September 22, 2020 Abstract How the single virus is forceful and shakes the world is eyewitness during this contemporary COVID 19 pandemic time. People primarily think of viruses such as HIV, Ebola, Zika, Influenza, Tobacco mosaic virus or whatever new outbreak like SARS, Corona are Understandingall viruses worst the and microbial non-beneficial. world isHowever, very critical not all and viruses crucial are thing detrimental that they and are influential driving force to human, and governing animal and the plantphysical health. world In fact, some viruses have beneficial properties for their hosts in a symbiotic relationship and scientific research in many disciplines. industry,and biosphere agriculture, at all. Thehealth virus and and environment other microbial are the life application those of bacteria, of microbes fungi, andprion, their viroid, products virion are are too requiring high for great human attention being and researchenvironment. to enhance Without their microbes utilization all lifefrom would majority be cease of useful on earth.aspects However, of microbial some genetic microbes resource. are very The dangerous secret behind like of Corona every virus, HIV, Ebola, Mycobacterium and others that destroy human life, but majority of microorganisms are too useful to promote virusdevelopment. in respect Through with health, building environment, and strengthening agriculture microbial and biotechnological culture collection application centers during and through this Covid19 strong pandemic conservation time strategy, to raise awarenessit is possible about to exploit virus atmore all. -

An Insect Nidovirus Emerging from a Primary Tropical Rainforest
RESEARCH ARTICLE An Insect Nidovirus Emerging from a Primary Tropical Rainforest Florian Zirkel,a,b,c Andreas Kurth,d Phenix-Lan Quan,b Thomas Briese,b Heinz Ellerbrok,d Georg Pauli,d Fabian H. Leendertz,c W. Ian Lipkin,b John Ziebuhr,e Christian Drosten,a and Sandra Junglena,c Institute of Virology, University of Bonn Medical Center, Bonn, Germanya; Center for Infection and Immunity, Mailman School of Public Health, Columbia University, New York, New York, USAb; Research Group Emerging Zoonosesc and Center for Biological Safety-1,d Robert Koch Institute, Berlin, Germany; and Institute of Medical Virology, Justus Liebig University Gießen, Gießen, Germanye ABSTRACT Tropical rainforests show the highest level of terrestrial biodiversity and may be an important contributor to micro- bial diversity. Exploitation of these ecosystems may foster the emergence of novel pathogens. We report the discovery of the first insect-associated nidovirus, tentatively named Cavally virus (CAVV). CAVV was found with a prevalence of 9.3% during a sur- vey of mosquito-associated viruses along an anthropogenic disturbance gradient in Côte d’Ivoire. Analysis of habitat-specific virus diversity and ancestral state reconstruction demonstrated an origin of CAVV in a pristine rainforest with subsequent spread into agriculture and human settlements. Virus extension from the forest was associated with a decrease in virus diversity (P < 0.01) and an increase in virus prevalence (P < 0.00001). CAVV is an enveloped virus with large surface projections. The RNA genome comprises 20,108 nucleotides with seven major open reading frames (ORFs). ORF1a and -1b encode two large pro- teins that share essential features with phylogenetically higher representatives of the order Nidovirales, including the families Coronavirinae and Torovirinae, but also with families in a basal phylogenetic relationship, including the families Roniviridae and Arteriviridae. -

Synthesis of Potato Virus X Rnas by Membrane- Containing Extracts
JOURNAL OF VIROLOGY, July 1996, p. 4795–4799 Vol. 70, No. 7 0022-538X/96/$04.0010 Copyright q 1996, American Society for Microbiology Synthesis of Potato Virus X RNAs by Membrane- Containing Extracts SERGEY V. DORONIN AND CYNTHIA HEMENWAY* Department of Biochemistry, North Carolina State University, Raleigh, North Carolina 27695-7622 Received 16 October 1995/Accepted 30 March 1996 Membrane-containing extracts isolated from tobacco plants infected with the plus-strand RNA virus, potato virus X (PVX), supported synthesis of four major, high-molecular-weight PVX RNA products (R1 to R4). Nuclease digestion and hybridization studies indicated that R1 and R2 are a mixture of partially single- stranded replicative intermediates and double-stranded replicative forms. R3 and R4 are double-stranded products containing sequences typical of the two major PVX subgenomic RNAs. The newly synthesized RNAs were demonstrated to have predominantly plus-strand polarity. Synthesis of these products was remarkably stable in the presence of ionic detergents. Potato virus X (PVX), the type member of the Potexvirus tracts were derived from Nicotiana tabacum leaves at 7 days genus, is a flexuous rod-shaped particle containing a single, postinoculation by a procedure similar to that described by genomic RNA of 6.4 kb that is capped and polyadenylated (21, Lurie and Hendrix (15). Inoculated and upper leaves (50 g) 27). Of the five open reading frames (ORFs), the first encodes were homogenized in 150 ml of buffer I (50 mM Tris-HCl [pH a 165-kDa protein (P1) that has homology to other known 7.5], 250 mM sucrose, 5 mM MgCl2, 1 mM EDTA, 10 mM RNA-dependent RNA polymerase (RdRp) proteins (23). -

Small Hydrophobic Viral Proteins Involved in Intercellular Movement of Diverse Plant Virus Genomes Sergey Y
AIMS Microbiology, 6(3): 305–329. DOI: 10.3934/microbiol.2020019 Received: 23 July 2020 Accepted: 13 September 2020 Published: 21 September 2020 http://www.aimspress.com/journal/microbiology Review Small hydrophobic viral proteins involved in intercellular movement of diverse plant virus genomes Sergey Y. Morozov1,2,* and Andrey G. Solovyev1,2,3 1 A. N. Belozersky Institute of Physico-Chemical Biology, Moscow State University, Moscow, Russia 2 Department of Virology, Biological Faculty, Moscow State University, Moscow, Russia 3 Institute of Molecular Medicine, Sechenov First Moscow State Medical University, Moscow, Russia * Correspondence: E-mail: [email protected]; Tel: +74959393198. Abstract: Most plant viruses code for movement proteins (MPs) targeting plasmodesmata to enable cell-to-cell and systemic spread in infected plants. Small membrane-embedded MPs have been first identified in two viral transport gene modules, triple gene block (TGB) coding for an RNA-binding helicase TGB1 and two small hydrophobic proteins TGB2 and TGB3 and double gene block (DGB) encoding two small polypeptides representing an RNA-binding protein and a membrane protein. These findings indicated that movement gene modules composed of two or more cistrons may encode the nucleic acid-binding protein and at least one membrane-bound movement protein. The same rule was revealed for small DNA-containing plant viruses, namely, viruses belonging to genus Mastrevirus (family Geminiviridae) and the family Nanoviridae. In multi-component transport modules the nucleic acid-binding MP can be viral capsid protein(s), as in RNA-containing viruses of the families Closteroviridae and Potyviridae. However, membrane proteins are always found among MPs of these multicomponent viral transport systems.