14.8 Ester Hydrolysis 14,7 Thioesters
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Alkyldihydropyrones, New Polyketides Synthesized by a Type III Polyketide Synthase from Streptomyces Reveromyceticus
The Journal of Antibiotics (2014) 67, 819–823 & 2014 Japan Antibiotics Research Association All rights reserved 0021-8820/14 www.nature.com/ja ORIGINAL ARTICLE Alkyldihydropyrones, new polyketides synthesized by a type III polyketide synthase from Streptomyces reveromyceticus Teruki Aizawa1, Seung-Young Kim1, Shunji Takahashi2,3, Masahiko Koshita1, Mioka Tani1, Yushi Futamura3, Hiroyuki Osada2,3 and Nobutaka Funa1 Genome sequencing allows a rapid and efficient identification of novel catalysts that produce novel secondary metabolites. Here we describe the catalytic properties of dihydropyrone synthase A (DpyA), a novel type III polyketide synthase encoded in a linear plasmid of Streptomyces reveromyceticus. Heterologous expression of dpyA led to the accumulation of alkyldihydropyrones A (1), B (2), C (3) and D (4), which are novel dihydropyran compounds that exhibit weak cytotoxicity against the leukemia cell line HL-60. DpyA catalyzes the condensation of b-hydroxyl acid thioester and methylmalonyl-CoA to yield a triketide intermediate that then undergoes lactonization of a secondary alcohol and a thioester to give alkyldihydropyrone. The Journal of Antibiotics (2014) 67, 819–823; doi:10.1038/ja.2014.80; published online 2 July 2014 INTRODUCTION Very recently, Tang et al.11 discovered that presulficidin, which is Type III polyketide synthase (PKS), which is widely distributed synthesized by Cpz6, a type III PKS from the caprazamycin among higher plants, fungi and bacteria, catalyzes the assembly of biosynthetic cluster, relays sulfonate from 30-phosphoadenine-50- primary metabolites such as acyl-CoA compounds to synthesize phosphosulfate to caprazamycin. structurally complex polyketides.1 The reaction catalyzed by type III Genome sequence analyses of Streptomyces species have shown that PKS starts with the formation of a thioester bond between the the number of biosynthetic gene clusters encoded on the chromosome catalytic center cysteine and an acyl group derived from a starter is much higher than the number of secondary metabolites isolated substrate. -

The Reaction of Aminonitriles with Aminothiols: a Way to Thiol-Containing Peptides and Nitrogen Heterocycles in the Primitive Earth Ocean
life Article The Reaction of Aminonitriles with Aminothiols: A Way to Thiol-Containing Peptides and Nitrogen Heterocycles in the Primitive Earth Ocean Ibrahim Shalayel , Seydou Coulibaly, Kieu Dung Ly, Anne Milet and Yannick Vallée * Univ. Grenoble Alpes, CNRS, Département de Chimie Moléculaire, Campus, F-38058 Grenoble, France; [email protected] (I.S.); [email protected] (S.C.); [email protected] (K.D.L.); [email protected] (A.M.) * Correspondence: [email protected] Received: 28 September 2018; Accepted: 18 October 2018; Published: 19 October 2018 Abstract: The Strecker reaction of aldehydes with ammonia and hydrogen cyanide first leads to α-aminonitriles, which are then hydrolyzed to α-amino acids. However, before reacting with water, these aminonitriles can be trapped by aminothiols, such as cysteine or homocysteine, to give 5- or 6-membered ring heterocycles, which in turn are hydrolyzed to dipeptides. We propose that this two-step process enabled the formation of thiol-containing dipeptides in the primitive ocean. These small peptides are able to promote the formation of other peptide bonds and of heterocyclic molecules. Theoretical calculations support our experimental results. They predict that α-aminonitriles should be more reactive than other nitriles, and that imidazoles should be formed from transiently formed amidinonitriles. Overall, this set of reactions delineates a possible early stage of the development of organic chemistry, hence of life, on Earth dominated by nitriles and thiol-rich peptides (TRP). Keywords: origin of life; prebiotic chemistry; thiol-rich peptides; cysteine; aminonitriles; imidazoles 1. Introduction In ribosomes, peptide bonds are formed by the reaction of the amine group of an amino acid with an ester function. -

The Relative Rates of Thiol–Thioester Exchange and Hydrolysis for Alkyl and Aryl Thioalkanoates in Water
Orig Life Evol Biosph (2011) 41:399–412 DOI 10.1007/s11084-011-9243-4 PREBIOTIC CHEMISTRY The Relative Rates of Thiol–Thioester Exchange and Hydrolysis for Alkyl and Aryl Thioalkanoates in Water Paul J. Bracher & Phillip W. Snyder & Brooks R. Bohall & George M. Whitesides Received: 14 April 2011 /Accepted: 16 June 2011 / Published online: 5 July 2011 # Springer Science+Business Media B.V. 2011 Abstract This article reports rate constants for thiol–thioester exchange (kex), and for acid- mediated (ka), base-mediated (kb), and pH-independent (kw) hydrolysis of S-methyl thioacetate and S-phenyl 5-dimethylamino-5-oxo-thiopentanoate—model alkyl and aryl thioalkanoates, respectively—in water. Reactions such as thiol–thioester exchange or aminolysis could have generated molecular complexity on early Earth, but for thioesters to have played important roles in the origin of life, constructive reactions would have needed to compete effectively with hydrolysis under prebiotic conditions. Knowledge of the kinetics of competition between exchange and hydrolysis is also useful in the optimization of systems where exchange is used in applications such as self-assembly or reversible binding. For the alkyl thioester S-methyl thioacetate, which has been synthesized in −5 −1 −1 −1 −1 −1 simulated prebiotic hydrothermal vents, ka = 1.5×10 M s , kb = 1.6×10 M s , and −8 −1 kw = 3.6×10 s . At pH 7 and 23°C, the half-life for hydrolysis is 155 days. The second- order rate constant for thiol–thioester exchange between S-methyl thioacetate and 2- −1 −1 sulfonatoethanethiolate is kex = 1.7 M s . -

Appendix H EPA Hazardous Waste Law
Appendix H EPA Hazardous Waste Law This Appendix is intended to give you background information on hazardous waste laws and how they apply to you. For most U.S. Environmental Protection Agency (EPA) requirements that apply to the University, the Safety Department maintains compliance through internal inspections, record keeping and proper disposal. In Wisconsin, the Department of Natural Resources (DNR) has adopted the EPA regulations, consequently EPA and DNR regulations are nearly identical. EPA defines This Appendix only deals with "hazardous waste" as defined by the EPA. hazardous waste as Legally, EPA defines hazardous waste as certain hazardous chemical waste. This hazardous chemical Appendix does not address other types of regulated laboratory wastes, such as waste; radioactive, infectious, biological, radioactive or sharps. Chapter 8 descibes disposal procedures infectious and biohazardous waste for animals. Chapter 9 describes disposal procedures for sharps and other waste that are regulated by can puncture tissue. Chapter 11 discusses Radiation and the Radiation Safety for other agencies. Radiation Workers provides guidelines for the disposal of radioactive waste. Procedures for medical waste are written by the UW Hospital Safety Officer. The Office of Biological Safety can provide guidance for the disposal of infectious and biological waste. EPA regulations focus on industrial waste streams. As a result, many laboratory chemical wastes are not regulated by EPA as hazardous chemical waste. However, many unregulated chemical wastes do merit special handling and disposal If a waste can be procedures. Thus, Chapter 7 and Appendix A of this Guide recommend disposal defined as: procedures for many unregulated wastes as if they were EPA hazardous waste. -

8.6 Acidity of Alcohols and Thiols 355
08_BRCLoudon_pgs5-1.qxd 12/8/08 11:05 AM Page 355 8.6 ACIDITY OF ALCOHOLS AND THIOLS 355 ural barrier to the passage of ions. However, the hydrocarbon surface of nonactin allows it to enter readily into, and pass through, membranes. Because nonactin binds and thus transports ions, the ion balance crucial to proper cell function is upset, and the cell dies. Ion Channels Ion channels, or “ion gates,” provide passageways for ions into and out of cells. (Recall that ions are not soluble in membrane phospholipids.) The flow of ions is essen- tial for the transmission of nerve impulses and for other biological processes. A typical chan- nel is a large protein molecule imbedded in a cell membrane. Through various mechanisms, ion channels can be opened or closed to regulate the concentration of ions in the interior of the cell. Ions do not diffuse passively through an open channel; rather, an open channel contains regions that bind a specific ion. Such an ion is bound specifically within the channel at one side of the membrane and is somehow expelled from the channel on the other side. Remark- ably, the structures of the ion-binding regions of these channels have much in common with the structures of ionophores such as nonactin. The first X-ray crystal structure of a potassium- ion channel was determined in 1998 by a team of scientists at Rockefeller University led by Prof. Roderick MacKinnon (b. 1956), who shared the 2003 Nobel Prize in Chemistry for this work. The interior of the channel contains binding sites for two potassium ions; these sites are oxygen-rich, much like the interior of nonactin. -

Shale Gas and Groundwater Quality
Shale Gas and Groundwater Quality A literature review on fate and effects of added chemicals Alette Langenhoff 1202141-008 © Deltares, 2011 1202141-008-ZWS-0001, 28 December 2011, final Contents 1 Introduction 1 2 The process of fracturing or fracking 5 3 The use of chemicals 7 4 Polyacrylamide 8 4.1 Aerobic degradation of polyacrylamide 8 4.2 Anaerobic degradation 10 4.3 Chemical or physical removal 10 4.4 Conclusion on removal of polyacrylamide 10 5 Glutaraldehyde 12 5.1 Biocide 12 5.2 Biodegradation 12 5.3 Chemical inactivation of glutaraldehyde 13 6 Conclusions 14 7 References 15 Appendices 17 Appendices A Chemicals identified in hydraulic fracturing fluid and flowback/produced water (EPA, 2011). A-1 B Fracturing fluid ingredients and common uses (Europe Unconventional Gas 2011) B-1 C Properties of Polyacrylamide (source: Wikipedia) C-1 D Properties of Glutaraldehyde (source: Wikipedia) D-1 Shale Gas and Groundwater Quality i 1202141-008-ZWS-0001, 28 December 2011, final 1 Introduction Shale gas is a so-called unconventional sources of natural gas, and is one of the most rapidly expanding trends in onshore domestic oil and gas exploration and production today (Fig. 1 and 2). Shale gas is present in hydrocarbon rich shale formations. Shallow gas is commonly defined as gas occurrences in unconsolidated sediments of Tertiary age (often down to depths of 1000 m below surface). The occurrences are positively associated with thick Neogene sediments and are often trapped in anticlinal structures associated with rising salt domes (Muntendam-Bos et al, 2009). Shale has low matrix permeability, so gas production in commercial quantities requires fractures to provide permeability. -

APPENDIX G Acid Dissociation Constants
harxxxxx_App-G.qxd 3/8/10 1:34 PM Page AP11 APPENDIX G Acid Dissociation Constants § ϭ 0.1 M 0 ؍ (Ionic strength ( † ‡ † Name Structure* pKa Ka pKa ϫ Ϫ5 Acetic acid CH3CO2H 4.756 1.75 10 4.56 (ethanoic acid) N ϩ H3 ϫ Ϫ3 Alanine CHCH3 2.344 (CO2H) 4.53 10 2.33 ϫ Ϫ10 9.868 (NH3) 1.36 10 9.71 CO2H ϩ Ϫ5 Aminobenzene NH3 4.601 2.51 ϫ 10 4.64 (aniline) ϪO SNϩ Ϫ4 4-Aminobenzenesulfonic acid 3 H3 3.232 5.86 ϫ 10 3.01 (sulfanilic acid) ϩ NH3 ϫ Ϫ3 2-Aminobenzoic acid 2.08 (CO2H) 8.3 10 2.01 ϫ Ϫ5 (anthranilic acid) 4.96 (NH3) 1.10 10 4.78 CO2H ϩ 2-Aminoethanethiol HSCH2CH2NH3 —— 8.21 (SH) (2-mercaptoethylamine) —— 10.73 (NH3) ϩ ϫ Ϫ10 2-Aminoethanol HOCH2CH2NH3 9.498 3.18 10 9.52 (ethanolamine) O H ϫ Ϫ5 4.70 (NH3) (20°) 2.0 10 4.74 2-Aminophenol Ϫ 9.97 (OH) (20°) 1.05 ϫ 10 10 9.87 ϩ NH3 ϩ ϫ Ϫ10 Ammonia NH4 9.245 5.69 10 9.26 N ϩ H3 N ϩ H2 ϫ Ϫ2 1.823 (CO2H) 1.50 10 2.03 CHCH CH CH NHC ϫ Ϫ9 Arginine 2 2 2 8.991 (NH3) 1.02 10 9.00 NH —— (NH2) —— (12.1) CO2H 2 O Ϫ 2.24 5.8 ϫ 10 3 2.15 Ϫ Arsenic acid HO As OH 6.96 1.10 ϫ 10 7 6.65 Ϫ (hydrogen arsenate) (11.50) 3.2 ϫ 10 12 (11.18) OH ϫ Ϫ10 Arsenious acid As(OH)3 9.29 5.1 10 9.14 (hydrogen arsenite) N ϩ O H3 Asparagine CHCH2CNH2 —— —— 2.16 (CO2H) —— —— 8.73 (NH3) CO2H *Each acid is written in its protonated form. -

Identification of a Thioesterase Bottleneck in the Pikromycin Pathway Through Full-Module Processing of Unnatural Pentaketides
Article pubs.acs.org/JACS Identification of a Thioesterase Bottleneck in the Pikromycin Pathway through Full-Module Processing of Unnatural Pentaketides † ‡ † § † ‡ ⊥ ∥ Douglas A. Hansen, , Aaron A. Koch, , and David H. Sherman*, , , , † ‡ § ⊥ Life Sciences Institute, Department of Medicinal Chemistry, Cancer Biology Graduate Program, Department of Chemistry, and ∥ Department of Microbiology & Immunology, University of Michigan, Ann Arbor, Michigan 48109, United States *S Supporting Information ABSTRACT: Polyketide biosynthetic pathways have been engineered to generate natural product analogs for over two decades. However, manipulation of modular type I polyketide synthases (PKSs) to make unnatural metabolites commonly results in attenuated yields or entirely inactive pathways, and the mechanistic basis for compromised production is rarely elucidated since rate-limiting or inactive domain(s) remain unidentified. Accordingly, we synthesized and assayed a series of modified pikromycin (Pik) pentaketides that mimic early pathway engineering to probe the substrate tolerance of the PikAIII-TE module in vitro. Truncated pentaketides were processed with varying efficiencies to corresponding macrolactones, while pentaketides with epimerized chiral centers were poorly processed by PikAIII-TE and failed to generate 12-membered ring products. Isolation and identification of extended but prematurely offloaded shunt products suggested that the Pik thioesterase (TE) domain has limited substrate flexibility and functions as a gatekeeper in the processing of -
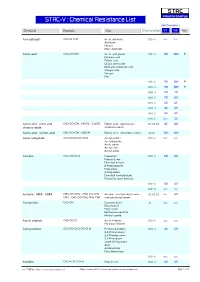
STAC-V : Chemical Resistance List Max Temperature
S TA C Industrial Coatings STAC-V : Chemical Resistance List Max Temperature Chemical Formula Alias Concentration V1 V2 Note Acetaldehyde CH3-CH=O Acetic aldehyde 100 % n.r. n.r. Aldehyde Ethanal Ethyl aldehyde Acetic acid CH3-CO-OH Acetic acid glacial 010 % 90 100 0 Ethanoic acid Ethylic acid Glacial acetic acid Methane carboxylic acid Vinegar acid Vinegar Hac 015 % 90 100 0 025 % 90 100 0 040 % 80 90 050 % 70 80 075 % 60 65 080 % 45 45 085 % 45 45 100 % n.r. 25 Acetic acid : nitric acid : CH3-CO-OH : HNO3 : Cr2O3 Ethylic acid : salpeterzuur : 03:05:03 65 80 chromic oxide chromium oxide Acetic acid : sulfuric acid CH3-CO-OH : H2SO4 Ethylic acid : dihydrogen sulfate 20:10 100 100 Acetic anhydride CH3-CO-O-CO-CH3 Acetyl acetate 100 % n.r. n.r. Acetanhydride Acetic oxide Acetyl ether Acetyl oxide Acetone CH3-CO-CH3 Propanone 005 % 80 80 Propan-2-one Dimethyl ketone β-Ketopropane[ Propanone 2-Propanone Dimethyl formaldehyde Pyroacetic spirit (archaic) 010 % 80 80 100 % n.r. n.r. Acetone : MEK : MiBK CH3-CO-CH3 : CH3-CO-CH2- Acetone : methylethyl ketone : 02:02:02 n.r. 40 CH3 : CH3-CO-CH2-CH2-CH3 methylisobutyl ketone Acetonitrile CH3-CN Cyanomethane all n.r. n.r. Ethanenitrile Ethyl nitrile Methanecarbonitrile Methyl cyanid Acetyl chloride CH3-CO-Cl Acetic chloride 100 % n.r. n.r. Ethanoyl chloride Acetylacetone CH3-CO-CH2-CO-CH3 Pentane-2,4-dione 020 % 40 50 2,4-Pentanedione 2,4-Dioxopentane 2,4-Pentadione acetyl-2-Propanone Acac Acetoacetone Diacetylmethane 100 % n.r. -

Estimation of Hydrolysis Rate Constants of Carboxylic Acid Ester and Phosphate Ester Compounds in Aqueous Systems from Molecular Structure by SPARC
Estimation of Hydrolysis Rate Constants of Carboxylic Acid Ester and Phosphate Ester Compounds in Aqueous Systems from Molecular Structure by SPARC R E S E A R C H A N D D E V E L O P M E N T EPA/600/R-06/105 September 2006 Estimation of Hydrolysis Rate Constants of Carboxylic Acid Ester and Phosphate Ester Compounds in Aqueous Systems from Molecular Structure by SPARC By S. H. Hilal Ecosystems Research Division National Exposure Research Laboratory Athens, Georgia U.S. Environmental Protection Agency Office of Research and Development Washington, DC 20460 NOTICE The information in this document has been funded by the United States Environmental Protection Agency. It has been subjected to the Agency's peer and administrative review, and has been approved for publication. Mention of trade names of commercial products does not constitute endorsement or recommendation for use. ii ABSTRACT SPARC (SPARC Performs Automated Reasoning in Chemistry) chemical reactivity models were extended to calculate hydrolysis rate constants for carboxylic acid ester and phosphate ester compounds in aqueous non- aqueous and systems strictly from molecular structure. The energy differences between the initial state and the transition state for a molecule of interest are factored into internal and external mechanistic perturbation components. The internal perturbations quantify the interactions of the appended perturber (P) with the reaction center (C). These internal perturbations are factored into SPARC’s mechanistic components of electrostatic and resonance effects. External perturbations quantify the solute-solvent interactions (solvation energy) and are factored into H-bonding, field stabilization and steric effects. These models have been tested using 1471 reliable measured base, acid and general base-catalyzed carboxylic acid ester hydrolysis rate constants in water and in mixed solvent systems at different temperatures. -

THIOL OXIDATION a Slippery Slope the Oxidation of Thiols — Molecules RSH Oxidation May Proceed Too Predominates
RESEARCH HIGHLIGHTS Nature Reviews Chemistry | Published online 25 Jan 2017; doi:10.1038/s41570-016-0013 THIOL OXIDATION A slippery slope The oxidation of thiols — molecules RSH oxidation may proceed too predominates. Here, the maximum of the form RSH — can afford quickly for intermediates like RSOH rate constants indicate the order − − − These are many products. From least to most to be spotted and may also afford of reactivity: RSO > RS >> RSO2 . common oxidized, these include disulfides intractable mixtures. Addressing When the reactions are carried out (RSSR), as well as sulfenic (RSOH), the first problem, Chauvin and Pratt in methanol-d , the obtained kinetic reactions, 1 sulfinic (RSO2H) and sulfonic slowed the reactions down by using isotope effect values (kH/kD) are all but have (RSO3H) acids. Such chemistry “very sterically bulky thiols, whose in the range 1.1–1.2, indicating that historically is pervasive in nature, in which corresponding sulfenic acids were no acidic proton is transferred in the been very disulfide bonds between cysteine known to be isolable but were yet rate-determining step. Rather, the residues stabilize protein structures, to be thoroughly explored in terms oxidations involve a specific base- difficult to and where thiols and thiolates often of reactivity”. The second problem catalysed mechanism wherein an study undergo oxidation by H2O2 or O2 in was tackled by modifying the model acid–base equilibrium precedes the order to protect important biological system, 9-mercaptotriptycene, by rate-determining nucleophilic attack − − − structures from damage. Among including a fluorine substituent to of RS , RSO or RSO2 on H2O2. the oxidation products, sulfenic serve as a spectroscopic handle. -

Hydrolysis and Atmospheric Oxidation Reactions of Perfluorinated Carboxylic Acid Precursors
Hydrolysis and Atmospheric Oxidation Reactions of Perfluorinated Carboxylic Acid Precursors by Derek A. Jackson A thesis submitted in conformity with the requirements for the degree of Doctor of Philosophy Graduate Department of Chemistry University of Toronto © Copyright by Derek A. Jackson 2013 Hydrolysis and Atmospheric Oxidation Reactions of Perfluorinated Carboxylic Acid Precursors Derek Andrew Jackson Doctor of Philosophy Department of Chemistry University of Toronto 2013 Abstract This dissertation explores a number of different environmentally relevant reactions that lead to the production of perfluorocarboxylic acids (PFCAs), a family of environmental pollutants that does not undergo any further degradation pathways. The compound perfluoro-2-methyl-3-pentanone (PFMP) is a new fire fighting fluid developed by 3M that is designed as a Halon replacement. The environment fate of PFMP with regards to direct photolysis, abiotic hydrolysis and hydration was determined using a combination of laboratory experiments and computational modeling. PFMP was found to undergo direct photolysis giving a lifetime of 4-14 days depending on latitude and time of year. Offline samples confirmed PFCA products and a mechanism was proposed. Polyfluorinated amides (PFAMs) are a class of chemicals produced as byproducts of polyfluorinated sulfonamide synthesis via electrochemical fluorination (ECF). Using synthesized standards of four model compounds, PFAMs were detected and quantified in a variety of legacy commercial materials synthesized by ECF. PFAMs were hypothesized to undergo biological hydrolysis reactions, suggesting their importance as historical PFOA precursors. ii The PFAMs were also investigated with regards to their environmental fate upon atmospheric oxidation. Using a smog chamber, the kinetics and degradation mechanisms of N- ethylperfluorobutyramide (EtFBA) were elucidated.