) (51) International Patent Classification: Declarations Under Rule 4.17: — As to Applicant's Entitlement to Applyfor and Be G
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Standard X-Ray Diffraction Powder Patterns
NBS MONOGRAPH 25 — SECTION 1 Standard X-ray Diffraction U.S. DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS THE NATIONAL BUREAU OF STANDARDS Functions and Activities The functions of the National Bureau of Standards are set forth in the Act of Congress, March 3, 1901, as amended by Congress in Public Law 619, 1950. These include the development and maintenance of the national standards of measurement and the provision of means and methods for making measurements consistent with these standards; the determination of physical constants and properties of materials; the development of methods and instruments for testing materials, devices, and structures; advisory services to government agencies on scien- tific and technical problems; invention and development of devices to serve special needs of the Government; and the development of standard practices, codes, and specifications. The work includes basic and applied research, development, engineering, instrumentation, testing, evaluation, calibration services, and various consultation and information services. Research projects are also performed for other government agencies when the work relates to and supplements the basic program of the Bureau or when the Bureau's unique competence is required. The scope of activities is suggested by the listing of divisions and sections on the inside of the back cover. Publications The results of the Bureau's research are published either in the Bureau's own series of publications or in the journals of professional and scientific societies. The Bureau itself publishes three periodicals available from the Government Printing Office: The Journal of Research, published in four separate sections, presents complete scientific and technical papers; the Technical News Bulletin presents summary and preliminary reports on work in progress; and Basic Radio Propagation Predictions provides data for determining the best frequencies to use for radio communications throughout the world. -

Appendix on Tariff Elimination Schedule for Mercosur
Trade part of the EU-Mercosur Association Agreement Without Prejudice Disclaimer: In view of the Commission's transparency policy, the Commission is publishing the texts of the Trade Part of the Agreement following the agreement in principle announced on 28 June 2019. The texts are published for information purposes only and may undergo further modifications including as a result of the process of legal revision. However, in view of the growing public interest in the negotiations, the texts are published at this stage of the negotiations for information purposes. These texts are without prejudice to the final outcome of the agreement between the EU and Mercosur. The texts will be final upon signature. The agreement will become binding on the Parties under international law only after completion by each Party of its internal legal procedures necessary for the entry into force of the Agreement (or its provisional application). AR applied BR applied PY applied UY applied Mercosur Final NCM Description Comments tariff tariff tariff tariff Offer 01012100 Pure-bred horses 0 0 0 0 0 01012900 Lives horses, except pure-bred breeding 2 2 2 2 0 01013000 Asses, pure-bred breeding 4 4 4 4 4 01019000 Asses, except pure-bred breeding 4 4 4 4 4 01022110 Purebred breeding cattle, pregnant or lactating 0 0 0 0 0 01022190 Other pure-bred cattle, for breeding 0 0 0 0 0 01022911 Other bovine animals for breeding,pregnant or lactating 2 2 2 2 0 01022919 Other bovine animals for breeding 2 2 2 2 4 01022990 Other live catlle 2 2 2 2 0 01023110 Pure-bred breeding buffalo, pregnant or lactating 0 0 0 0 0 01023190 Other pure-bred breeding buffalo 0 0 0 0 0 01023911 Other buffalo for breeding, ex. -

Tonsillopharyngitis - Acute (1 of 10)
Tonsillopharyngitis - Acute (1 of 10) 1 Patient presents w/ sore throat 2 EVALUATION Yes EXPERT Are there signs of REFERRAL complication? No 3 4 EVALUATION Is Group A Beta-hemolytic Yes DIAGNOSIS Streptococcus (GABHS) • Rapid antigen detection test infection suspected? (RADT) • roat culture No TREATMENT EVALUATION No A Supportive management Is GABHS confi rmed? B Pharmacological therapy (Non-GABHS) Yes 5 TREATMENT A EVALUATE RESPONSEMIMS Supportive management TO THERAPY C Pharmacological therapy • Antibiotics Poor/No Good D Surgery, if recurrent or complicated response response REASSESS PATIENT COMPLETE THERAPY & REVIEW THE DIAGNOSIS© Not all products are available or approved for above use in all countries. Specifi c prescribing information may be found in the latest MIMS. B269 © MIMS Pediatrics 2020 Tonsillopharyngitis - Acute (2 of 10) 1 ACUTE TONSILLOPHARYNGITIS • Infl ammation of the tonsils & pharynx • Etiologies include bacterial (group A β-hemolytic streptococcus, Haemophilus infl uenzae, Fusobacterium sp, etc) & viral (infl uenza, adenovirus, coronavirus, rhinovirus, etc) pathogens • Sore throat is the most common presenting symptom in older children TONSILLOPHARYNGITIS 2 EVALUATION FOR COMPLICATIONS • Patients w/ sore throat may have deep neck infections including epiglottitis, peritonsillar or retropharyngeal abscess • Examine for signs of upper airway obstruction Signs & Symptoms of Sore roat w/ Complications • Trismus • Inability to swallow liquids • Increased salivation or drooling • Peritonsillar edema • Deviation of uvula -

Health Reports for Mutual Recognition of Medical Prescriptions: State of Play
The information and views set out in this report are those of the author(s) and do not necessarily reflect the official opinion of the European Union. Neither the European Union institutions and bodies nor any person acting on their behalf may be held responsible for the use which may be made of the information contained therein. Executive Agency for Health and Consumers Health Reports for Mutual Recognition of Medical Prescriptions: State of Play 24 January 2012 Final Report Health Reports for Mutual Recognition of Medical Prescriptions: State of Play Acknowledgements Matrix Insight Ltd would like to thank everyone who has contributed to this research. We are especially grateful to the following institutions for their support throughout the study: the Pharmaceutical Group of the European Union (PGEU) including their national member associations in Denmark, France, Germany, Greece, the Netherlands, Poland and the United Kingdom; the European Medical Association (EMANET); the Observatoire Social Européen (OSE); and The Netherlands Institute for Health Service Research (NIVEL). For questions about the report, please contact Dr Gabriele Birnberg ([email protected] ). Matrix Insight | 24 January 2012 2 Health Reports for Mutual Recognition of Medical Prescriptions: State of Play Executive Summary This study has been carried out in the context of Directive 2011/24/EU of the European Parliament and of the Council of 9 March 2011 on the application of patients’ rights in cross- border healthcare (CBHC). The CBHC Directive stipulates that the European Commission shall adopt measures to facilitate the recognition of prescriptions issued in another Member State (Article 11). At the time of submission of this report, the European Commission was preparing an impact assessment with regards to these measures, designed to help implement Article 11. -

)&F1y3x PHARMACEUTICAL APPENDIX to THE
)&f1y3X PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE )&f1y3X PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 3 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. Product CAS No. Product CAS No. ABAMECTIN 65195-55-3 ACTODIGIN 36983-69-4 ABANOQUIL 90402-40-7 ADAFENOXATE 82168-26-1 ABCIXIMAB 143653-53-6 ADAMEXINE 54785-02-3 ABECARNIL 111841-85-1 ADAPALENE 106685-40-9 ABITESARTAN 137882-98-5 ADAPROLOL 101479-70-3 ABLUKAST 96566-25-5 ADATANSERIN 127266-56-2 ABUNIDAZOLE 91017-58-2 ADEFOVIR 106941-25-7 ACADESINE 2627-69-2 ADELMIDROL 1675-66-7 ACAMPROSATE 77337-76-9 ADEMETIONINE 17176-17-9 ACAPRAZINE 55485-20-6 ADENOSINE PHOSPHATE 61-19-8 ACARBOSE 56180-94-0 ADIBENDAN 100510-33-6 ACEBROCHOL 514-50-1 ADICILLIN 525-94-0 ACEBURIC ACID 26976-72-7 ADIMOLOL 78459-19-5 ACEBUTOLOL 37517-30-9 ADINAZOLAM 37115-32-5 ACECAINIDE 32795-44-1 ADIPHENINE 64-95-9 ACECARBROMAL 77-66-7 ADIPIODONE 606-17-7 ACECLIDINE 827-61-2 ADITEREN 56066-19-4 ACECLOFENAC 89796-99-6 ADITOPRIM 56066-63-8 ACEDAPSONE 77-46-3 ADOSOPINE 88124-26-9 ACEDIASULFONE SODIUM 127-60-6 ADOZELESIN 110314-48-2 ACEDOBEN 556-08-1 ADRAFINIL 63547-13-7 ACEFLURANOL 80595-73-9 ADRENALONE -

Non-Pharmacologic Therapies and Airway Clearance Techniques in Bronchiectasis
Division of Pulmonary, Critical Care and Sleep Medicine NON-PHARMACOLOGIC THERAPIES AND AIRWAY CLEARANCE TECHNIQUES IN BRONCHIECTASIS Ashwin Basavaraj, MD, FCCP Associate Director, NYU Bronchiectasis Program NTM Patient Education Program DC 11/24/2019 October 30, 2019 Financial Disclosure • Insmed - Consultant, Advisory Board (Active) • Hill-Rom – Consultant, Principal investigator on a clinical trial (Active) • COPD foundation grant on airway clearance 2 Division of Pulmonary, Critical Care and Sleep Medicine DC 11/24/2019 Case presentation • 66 year-old female with a history of prior pneumonia 15 years ago presents with productive cough. • She has mild shortness of breath. No fevers, no hemoptysis. She has gained two pounds over the year. • No other prior medical history, and currently not taking any medications • Initial workup including autoimmune serologies and quantitative immunoglobulin levels were negative. • You check AFB, bacterial and fungal sputum cultures. • She has 2 out 3 cultures positive for MAC. 3 Division of Pulmonary, Critical Care and Sleep Medicine DC 11/24/2019 4 Division of Pulmonary, Critical Care and Sleep Medicine DC 11/24/2019 What’s the next best step in management? A) Start 3 drug antibiotic therapy for MAC B) Initiate airway clearance with nebulized hypertonic saline and a positive expiratory pressure device C) Start antibiotics for MAC and initiate airway clearance D) Closely monitor without initiation of treatment 5 Division of Pulmonary, Critical Care and Sleep Medicine DC 11/24/2019 GOALS OF AIRWAY CLEARANCE Short term goals Long term goals • Provide more effective sputum • Reduce further airway damage by clearance that improves ventilation halting the vicious cycle • Reduce cough and breathlessness • Reduce pulmonary exacerbations • Improve quality of life O’Neill, et al. -

Management of Breathlessness in Patients with Life Limiting Disease
MANAGEMENT OF BREATHLESSNESS IN PATIENTS WITH LIFE LIMITING DISEASE Helen Armstrong Dr Helen Bonwick Dr Clare Jeffries Dr Martin Ledson Dr Kate Marley Mrs Sue Oakes CURRENT STANDARDS AND GUIDELINES • Current standards and guidleines • Literature review – pharmacological and non- pharmacological • Audit results • Proposed new standards and guidelines • Current management of cough Guidelines Non pharmacological options (Level 4 ) • These are important and should not be overlooked. They may be used alone or in conjunction with medication. • They include – Reassurance and explanation – Use of fan or cool air across face – Adequate positioning of the patient to aid breathing – Breathing exercises and relaxation training – Advice on modifying lifestyle – Acupuncture, aromatherapy and reflexology Guidelines Pharmacological options Benzodiazepines (Level 3) • Benzodiazepines may be useful especially if there is coexisting anxiety and/or fear. • Lorazepam is suggested for episodes of paroxysmal breathlessness. Dose: 0.5mg-1mg sublingually as required (max dose 4mg daily). • In patients unable to tolerate oral medication or those in the dying phase, subcutaneous Midazolam 2.5mg-5mg as required may be appropriate. If effective this can be incorporated into a 24hour subcutaneous infusion via syringe driver. Guidelines Nebulised Medication (Level 4)/(Level 1) NB: The first medication of any nebulised medication, including saline, must be monitored for adverse effect such as bronchospasm. • Nebulised non opioids – Nebulised sodium chloride 0.9% may help as a mucolytic. Consider trial for 24hours. Dose: 5ml via a nebuliser 4 hourly as required. – A trial of nebulised bronchodilator should be considered if there is evidence of airways obstruction (Level 4) commonly prescribed bronchdilators are Salbutamol and Ipratropium Bromide. -

Bahrain-Pharma-1444108170.Pdf
Fill Type of Competition S. No BP Brand Name Therapeutical Class Potency Generic Name/Composition Volume Dosage Name,company, retail price form Each 5 ml contents: Samol 120 ml Salbutamol Sulphate 2mg Salbutamol 1 Anti-Asthmatic agent (Bronchodilator) Each 5ml contains Acefylline piperazine 125mg Pifalin 100 ml Acefylline Piperazine 2 (125mg/5ml) Acefylline Piperazine 45mg, Diphenhydramine Dephicef 100 ml Acefylline Piperazine + Diphenhydramine 3 8mg/5ml Ammonium Chloride 100.00mg , Sodium Clomadrin 100 ml Citrate 60.00mg, Ephedrine HCl 7.00mg, Ammonium Chloride + Sodium Citrate + Ephedrine HCl + Chlorpheniramine Maleate 4 Chlorpheniramine Maleate 2.00mg/5ml Each 5 ml contents: Ambroxol HCl eq. to 100 ml Ambroxol 15mg 5 Ambrol Ambroxol HCl Each 5 ml contents: Ambroxol HCl eq. to Cough and Cold 100 ml Syrup 6 Ambroxol 30mg 7 Dextron 100 ml 15mg/5 ml Dextromethorphan Hbr 8 Gufosil 100 ml 100mg/5 ml Guaifenesin 9 Proligen 120 ml 1.25mg + 30mg/5 ml Triprolodin HCl + Pseudoephedrine HCl 10 Tripodil 120 ml 1.25mg + 30mg + 10mg/5 ml Triprolodin HCL + Pseudoephedrine HCl + Dextromethorphan Hbr 11 Tripogin 120 ml 1.25mg + 30mg + 100 mg/5 ml Triprolodin HCL + Pseudoephedrine HCl + Guaifenesin 12 Dexotrin 120 ml 1.25mg +10mg + 7.5mg + 50mg/5 ml Triprolodin HCL + Pseudoephedrine HCl + Dextromethorphan Hbr + Guaifenesin Wild cherry (Prunus serotina) + (myroxylon balsamum) + Mallow (Malva Sylvestris) + Welcosin 120 ml 60mg + 18.75mg + 7.5mg + 7.5mg / 5ml 13 Marshnallow (Althaea officinalis) 14 Ivosil 100 ml Each 5 ml contents: Ivy leaf 15 Hexobim 100 ml 4mg/5mlIvy leaves dried extract (4-8:1, 100%) 100 mg Bromhexine HCl Carbocisteine 2% and 5% (i.e. -
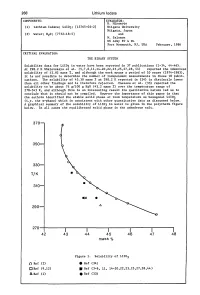
Evaluation of Aqueous Systems
268 Lithium Iodate COMPONENTS: EVALUATOR: H. Miyamoto (1) Lithium Iodate; LiI03; [13765-03-2] Niigata University Niigata, Japan (2) Water; H20; [7732-18-5] and M. Salomon US Army ET & DL Fort Monmouth, NJ, USA February, 1986 CRITICAL EVALUATION: THE BINARY SYSTEM Solubility data for LiI03 in water have been reported in 37 publications (1-34, 44-46). At 298.2 K Shklovskaya et al. (5,7,8,11,14-20,22,23,25,27,28,44) reported the identical solubility of 43.82 mass %, and although the work spans a period of 10 years (1974-1983), it is not possible to determine the number of independent measurements in these 18 publi cations. The solubility of 43.30 mass % at 298.2 K reported in (24) is distinctly lower than all other findings and is therefore rejected. Unezawa et al. (33) reported the solubility to be about 76 g/lOO g H20 (43.2 mass %) over the temperature range of 278-343 K, and although this is an interesting result its qualitative nature led us to conclude that it should not be compiled. However the importance of this paper is that the authors identified the stable solid phase at room temperature as hexagonal LiI03 (i.e. the a-phase) which is consistent with other quantitative data as discussed below. A graphical summary of the solubility of LiI03 in water is given in the polytherm figure below. In all cases the equilibrated solid phase is the anhydrous salt. 370 350 330 T/K 310 290 270-I------.r-----.-----..----......----------. 42 43 44 45 46 47 48 mass % Figure 1. -

(12) United States Patent (10) Patent No.: US 9,314.465 B2 Brew Et Al
US009314465B2 (12) United States Patent (10) Patent No.: US 9,314.465 B2 Brew et al. (45) Date of Patent: *Apr. 19, 2016 (54) DRUG COMBINATIONS AND USES IN 2008.0003280 A1 1/2008 Levine et al. ................. 424/456 TREATING A COUGHING CONDITION 2008/O176955 A1 7/2008 Hecket al. 2008, 0220078 A1 9, 2008 Morton et al. (71) Applicant: Infirst Healthcare Limited 2009, O136427 A1 5/2009 Croft et al. 2009, O220594 A1 9, 2009 Field (72) Inventors: John Brew, London (GB); Robin Mark 2012/O128738 A1 5, 2012 Brew et al. Bannister, London (GB) 2012fO252824 A1 10/2012 Brew et al. (73) Assignee: Infirst Healthcare Limited, London FOREIGN PATENT DOCUMENTS (GB) CN 1593451 3, 2005 CN 101024.014 A 8, 2007 (*) Notice: Subject to any disclaimer, the term of this CN 101112383 B 5, 2010 patent is extended or adjusted under 35 DE 4420708 A1 12, 1995 U.S.C. 154(b) by 0 days. EP 2050435 B1 4/2009 GB 2114001 A 8, 1983 This patent is Subject to a terminal dis GB 2284761 A 6, 1995 claimer. GB 2424.185 B 9, 2006 GB 2442828 A 4/2008 JP 62-249924 A 10, 1987 (21) Appl. No.: 14/287,014 JP H1O-316568 A 12/1998 JP 2001-518928 A 10, 2001 (22) Filed: May 24, 2014 JP 200219.3839. A T 2002 JP 2003-012514 A 1, 2003 (65) Prior Publication Data JP 20030552.58 A 2, 2003 JP 2003128549 A 5, 2003 US 2014/O256750 A1 Sep. 11, 2014 JP 2003-321357 A 11, 2003 JP 2005-516917 A 6, 2005 JP 2008O31146 A 2, 2008 Related U.S. -

UNIVERSITE DE NANTES Thomas Gelineau
UNIVERSITE DE NANTES __________ FACULTE DE MEDECINE __________ Année 2011 N° 139 THESE pour le DIPLÔME D’ÉTAT DE DOCTEUR EN MÉDECINE DES de médecine générale par Thomas Gelineau né le 29 janvier 1983 à Cholet __________ Présentée et soutenue publiquement le 06/12/2011 __________ LE RHUME DE L'ENFANT ET SON TRAITEMENT: DECISION PARTAGEE AVEC LES PARENTS D'APRES UN QUESTIONNAIRE __________ Président : Monsieur le Professeur Olivier MALARD Directeur de thèse : Madame le Professeur Jacqueline LACAILLE 1 Table des matières IIntroduction................................................................................................................ 7 IIDéfinition, état des connaissances...........................................................................8 1Le rhume.......................................................................................................................................8 APhysiopathologie............................................................................................................................. 8 aL'origine virale.................................................................................................................................... 8 bLa saisonnalité................................................................................................................................... 8 cL'âge de survenue.............................................................................................................................. 9 dLe sexe.................................................................................................................................................. -

Handbook on the Physics and Chemistry of Rare Earths Volume 9 Elsevier, 1987
Handbook on the Physics and Chemistry of Rare Earths volume 9 Elsevier, 1987 Edited by: Karl A. Gschneidner, Jr. and LeRoy Eyring ISBN: 978-0-444-87045-2 by kmno4 Handbook on the Physics and Chemistry of Rare Earths, edited by K.A. Gschneidner, Jr. and L. Eyring © Elsevier Science Publishers B.V., 1987 PREFACE Karl A. GSCHNEIDNER, Jr., and LeRoy EYRING o These elements perplex us in our rearches [sic], baffle us in our speculations, and haunt us in our very dreams. They stretch like an unknown sea before us- mocking, mystifying, and murmuring strange revelations and possibilities. Sir William Crookes (February 16, 1887) There are those who feel that the rare earth elements are destined to play an even greater role in our "high-tech" society in the future than they have in the past. This judgement is based upon the trend of increasing applications resulting from the electronic structures of these materials that lead to their unusual optical, magnetic, electrical and chemical properties so adaptable to the demands now being placed on materials. The "Handbook" seeks to provide topical reviews and compilations of critically reviewed data to aid in the design and fabrication of the required new materials. Four additional chapters in this tradition are the contents of this volume. The development of lasers containing rare earth species sparked an interest in glasses containing these elements. Reisfeld and J0rgensen discuss excited-state phenomena in vitreous rare-earth-containing substances. The description of the chemistry and crystal chemistry of complex inorganic compounds of the rare earths is continued by Niinist6 and Leskelfi from the previous volume.