Bahrain-Pharma-1444108170.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The National Drugs List
^ ^ ^ ^ ^[ ^ The National Drugs List Of Syrian Arab Republic Sexth Edition 2006 ! " # "$ % &'() " # * +$, -. / & 0 /+12 3 4" 5 "$ . "$ 67"5,) 0 " /! !2 4? @ % 88 9 3: " # "$ ;+<=2 – G# H H2 I) – 6( – 65 : A B C "5 : , D )* . J!* HK"3 H"$ T ) 4 B K<) +$ LMA N O 3 4P<B &Q / RS ) H< C4VH /430 / 1988 V W* < C A GQ ") 4V / 1000 / C4VH /820 / 2001 V XX K<# C ,V /500 / 1992 V "!X V /946 / 2004 V Z < C V /914 / 2003 V ) < ] +$, [2 / ,) @# @ S%Q2 J"= [ &<\ @ +$ LMA 1 O \ . S X '( ^ & M_ `AB @ &' 3 4" + @ V= 4 )\ " : N " # "$ 6 ) G" 3Q + a C G /<"B d3: C K7 e , fM 4 Q b"$ " < $\ c"7: 5) G . HHH3Q J # Hg ' V"h 6< G* H5 !" # $%" & $' ,* ( )* + 2 ا اوا ادو +% 5 j 2 i1 6 B J' 6<X " 6"[ i2 "$ "< * i3 10 6 i4 11 6! ^ i5 13 6<X "!# * i6 15 7 G!, 6 - k 24"$d dl ?K V *4V h 63[46 ' i8 19 Adl 20 "( 2 i9 20 G Q) 6 i10 20 a 6 m[, 6 i11 21 ?K V $n i12 21 "% * i13 23 b+ 6 i14 23 oe C * i15 24 !, 2 6\ i16 25 C V pq * i17 26 ( S 6) 1, ++ &"r i19 3 +% 27 G 6 ""% i19 28 ^ Ks 2 i20 31 % Ks 2 i21 32 s * i22 35 " " * i23 37 "$ * i24 38 6" i25 39 V t h Gu* v!* 2 i26 39 ( 2 i27 40 B w< Ks 2 i28 40 d C &"r i29 42 "' 6 i30 42 " * i31 42 ":< * i32 5 ./ 0" -33 4 : ANAESTHETICS $ 1 2 -1 :GENERAL ANAESTHETICS AND OXYGEN 4 $1 2 2- ATRACURIUM BESYLATE DROPERIDOL ETHER FENTANYL HALOTHANE ISOFLURANE KETAMINE HCL NITROUS OXIDE OXYGEN PROPOFOL REMIFENTANIL SEVOFLURANE SUFENTANIL THIOPENTAL :LOCAL ANAESTHETICS !67$1 2 -5 AMYLEINE HCL=AMYLOCAINE ARTICAINE BENZOCAINE BUPIVACAINE CINCHOCAINE LIDOCAINE MEPIVACAINE OXETHAZAINE PRAMOXINE PRILOCAINE PREOPERATIVE MEDICATION & SEDATION FOR 9*: ;< " 2 -8 : : SHORT -TERM PROCEDURES ATROPINE DIAZEPAM INJ. -

Appendix on Tariff Elimination Schedule for Mercosur
Trade part of the EU-Mercosur Association Agreement Without Prejudice Disclaimer: In view of the Commission's transparency policy, the Commission is publishing the texts of the Trade Part of the Agreement following the agreement in principle announced on 28 June 2019. The texts are published for information purposes only and may undergo further modifications including as a result of the process of legal revision. However, in view of the growing public interest in the negotiations, the texts are published at this stage of the negotiations for information purposes. These texts are without prejudice to the final outcome of the agreement between the EU and Mercosur. The texts will be final upon signature. The agreement will become binding on the Parties under international law only after completion by each Party of its internal legal procedures necessary for the entry into force of the Agreement (or its provisional application). AR applied BR applied PY applied UY applied Mercosur Final NCM Description Comments tariff tariff tariff tariff Offer 01012100 Pure-bred horses 0 0 0 0 0 01012900 Lives horses, except pure-bred breeding 2 2 2 2 0 01013000 Asses, pure-bred breeding 4 4 4 4 4 01019000 Asses, except pure-bred breeding 4 4 4 4 4 01022110 Purebred breeding cattle, pregnant or lactating 0 0 0 0 0 01022190 Other pure-bred cattle, for breeding 0 0 0 0 0 01022911 Other bovine animals for breeding,pregnant or lactating 2 2 2 2 0 01022919 Other bovine animals for breeding 2 2 2 2 4 01022990 Other live catlle 2 2 2 2 0 01023110 Pure-bred breeding buffalo, pregnant or lactating 0 0 0 0 0 01023190 Other pure-bred breeding buffalo 0 0 0 0 0 01023911 Other buffalo for breeding, ex. -

Theophylline-7-Acetic Acid
Theophylline-7-acetic acid sc-237085 Material Safety Data Sheet Hazard Alert Code Key: EXTREME HIGH MODERATE LOW Section 1 - CHEMICAL PRODUCT AND COMPANY IDENTIFICATION PRODUCT NAME Theophylline-7-acetic acid STATEMENT OF HAZARDOUS NATURE CONSIDERED A HAZARDOUS SUBSTANCE ACCORDING TO OSHA 29 CFR 1910.1200. NFPA FLAMMABILITY1 HEALTH2 HAZARD INSTABILITY0 SUPPLIER Santa Cruz Biotechnology, Inc. 2145 Delaware Avenue Santa Cruz, California 95060 800.457.3801 or 831.457.3800 EMERGENCY ChemWatch Within the US & Canada: 877-715-9305 Outside the US & Canada: +800 2436 2255 (1-800-CHEMCALL) or call +613 9573 3112 SYNONYMS C9-H10-N4-O4, "purine-7-acetic acid, 1, 2, 3, 6-tetrahydro-1, 3-dimethyl-2, 6-dioxo-", acefylline, acephylline, 7-(carboxymethyl)theophylline, "1, 2, 3, 6-tetrahydro-1, 3-dimethyl-2, 6-dioxopurine-7-acetic acid", "7-theophyllineacetic acid", "7-theophyllinylacetic acid", alkaloid Section 2 - HAZARDS IDENTIFICATION CHEMWATCH HAZARD RATINGS Min Max Flammability: 1 Toxicity: 2 Body Contact: 2 Min/Nil=0 Low=1 Reactivity: 1 Moderate=2 High=3 Chronic: 2 Extreme=4 1 of 8 CANADIAN WHMIS SYMBOLS EMERGENCY OVERVIEW RISK Harmful if swallowed. Irritating to eyes, respiratory system and skin. POTENTIAL HEALTH EFFECTS ACUTE HEALTH EFFECTS SWALLOWED ! Accidental ingestion of the material may be harmful; animal experiments indicate that ingestion of less than 150 gram may be fatal or may produce serious damage to the health of the individual. ! Xanthine derivatives may produce nausea, vomiting, anorexia, stomach pain, vomiting of blood and diarrhea. Protein in the urine, increased amounts of urine output, and increased excretion of renal tubular cells and red blood cells may also occur. -

Non-Pharmacologic Therapies and Airway Clearance Techniques in Bronchiectasis
Division of Pulmonary, Critical Care and Sleep Medicine NON-PHARMACOLOGIC THERAPIES AND AIRWAY CLEARANCE TECHNIQUES IN BRONCHIECTASIS Ashwin Basavaraj, MD, FCCP Associate Director, NYU Bronchiectasis Program NTM Patient Education Program DC 11/24/2019 October 30, 2019 Financial Disclosure • Insmed - Consultant, Advisory Board (Active) • Hill-Rom – Consultant, Principal investigator on a clinical trial (Active) • COPD foundation grant on airway clearance 2 Division of Pulmonary, Critical Care and Sleep Medicine DC 11/24/2019 Case presentation • 66 year-old female with a history of prior pneumonia 15 years ago presents with productive cough. • She has mild shortness of breath. No fevers, no hemoptysis. She has gained two pounds over the year. • No other prior medical history, and currently not taking any medications • Initial workup including autoimmune serologies and quantitative immunoglobulin levels were negative. • You check AFB, bacterial and fungal sputum cultures. • She has 2 out 3 cultures positive for MAC. 3 Division of Pulmonary, Critical Care and Sleep Medicine DC 11/24/2019 4 Division of Pulmonary, Critical Care and Sleep Medicine DC 11/24/2019 What’s the next best step in management? A) Start 3 drug antibiotic therapy for MAC B) Initiate airway clearance with nebulized hypertonic saline and a positive expiratory pressure device C) Start antibiotics for MAC and initiate airway clearance D) Closely monitor without initiation of treatment 5 Division of Pulmonary, Critical Care and Sleep Medicine DC 11/24/2019 GOALS OF AIRWAY CLEARANCE Short term goals Long term goals • Provide more effective sputum • Reduce further airway damage by clearance that improves ventilation halting the vicious cycle • Reduce cough and breathlessness • Reduce pulmonary exacerbations • Improve quality of life O’Neill, et al. -

Management of Breathlessness in Patients with Life Limiting Disease
MANAGEMENT OF BREATHLESSNESS IN PATIENTS WITH LIFE LIMITING DISEASE Helen Armstrong Dr Helen Bonwick Dr Clare Jeffries Dr Martin Ledson Dr Kate Marley Mrs Sue Oakes CURRENT STANDARDS AND GUIDELINES • Current standards and guidleines • Literature review – pharmacological and non- pharmacological • Audit results • Proposed new standards and guidelines • Current management of cough Guidelines Non pharmacological options (Level 4 ) • These are important and should not be overlooked. They may be used alone or in conjunction with medication. • They include – Reassurance and explanation – Use of fan or cool air across face – Adequate positioning of the patient to aid breathing – Breathing exercises and relaxation training – Advice on modifying lifestyle – Acupuncture, aromatherapy and reflexology Guidelines Pharmacological options Benzodiazepines (Level 3) • Benzodiazepines may be useful especially if there is coexisting anxiety and/or fear. • Lorazepam is suggested for episodes of paroxysmal breathlessness. Dose: 0.5mg-1mg sublingually as required (max dose 4mg daily). • In patients unable to tolerate oral medication or those in the dying phase, subcutaneous Midazolam 2.5mg-5mg as required may be appropriate. If effective this can be incorporated into a 24hour subcutaneous infusion via syringe driver. Guidelines Nebulised Medication (Level 4)/(Level 1) NB: The first medication of any nebulised medication, including saline, must be monitored for adverse effect such as bronchospasm. • Nebulised non opioids – Nebulised sodium chloride 0.9% may help as a mucolytic. Consider trial for 24hours. Dose: 5ml via a nebuliser 4 hourly as required. – A trial of nebulised bronchodilator should be considered if there is evidence of airways obstruction (Level 4) commonly prescribed bronchdilators are Salbutamol and Ipratropium Bromide. -

Medicines Regulations 1984 (SR 1984/143)
Reprint as at 1 August 2011 Medicines Regulations 1984 (SR 1984/143) David Beattie, Governor-General Order in Council At the Government House at Wellington this 5th day of June 1984 Present: His Excellency the Governor-General in Council Pursuant to section 105 of the Medicines Act 1981, and, in the case of Part 3 of the regulations, to section 62 of that Act, His Excellency the Governor-General, acting on the advice of the Minister of Health tendered after consultation with the organisations and bodies that ap- peared to the Minister to be representatives of persons likely to be substantially affected, and by and with the advice and consent of the Executive Council, hereby makes the following regulations. Contents Page 1 Title and commencement 5 Note Changes authorised by section 17C of the Acts and Regulations Publication Act 1989 have been made in this reprint. A general outline of these changes is set out in the notes at the end of this reprint, together with other explanatory material about this reprint. These regulations are administered by the Ministry of Health. 1 Reprinted as at Medicines Regulations 1984 1 August 2011 2 Interpretation 5 Part 1 Classification of medicines 3 Classification of medicines 11 Part 2 Standards 4 Standards for medicines, related products, medical 11 devices, cosmetics, and surgical dressings 5 Pharmacist may dilute medicine in particular case 12 6 Colouring substances [Revoked] 12 Part 3 Advertisements 7 Advertisements not to claim official approval 12 8 Advertisements for medicines 13 9 Advertisements -
![Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set](https://docslib.b-cdn.net/cover/8870/ehealth-dsi-ehdsi-v2-2-2-or-ehealth-dsi-master-value-set-1028870.webp)
Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set
MTC eHealth DSI [eHDSI v2.2.2-OR] eHealth DSI – Master Value Set Catalogue Responsible : eHDSI Solution Provider PublishDate : Wed Nov 08 16:16:10 CET 2017 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 1 of 490 MTC Table of Contents epSOSActiveIngredient 4 epSOSAdministrativeGender 148 epSOSAdverseEventType 149 epSOSAllergenNoDrugs 150 epSOSBloodGroup 155 epSOSBloodPressure 156 epSOSCodeNoMedication 157 epSOSCodeProb 158 epSOSConfidentiality 159 epSOSCountry 160 epSOSDisplayLabel 167 epSOSDocumentCode 170 epSOSDoseForm 171 epSOSHealthcareProfessionalRoles 184 epSOSIllnessesandDisorders 186 epSOSLanguage 448 epSOSMedicalDevices 458 epSOSNullFavor 461 epSOSPackage 462 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 2 of 490 MTC epSOSPersonalRelationship 464 epSOSPregnancyInformation 466 epSOSProcedures 467 epSOSReactionAllergy 470 epSOSResolutionOutcome 472 epSOSRoleClass 473 epSOSRouteofAdministration 474 epSOSSections 477 epSOSSeverity 478 epSOSSocialHistory 479 epSOSStatusCode 480 epSOSSubstitutionCode 481 epSOSTelecomAddress 482 epSOSTimingEvent 483 epSOSUnits 484 epSOSUnknownInformation 487 epSOSVaccine 488 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 3 of 490 MTC epSOSActiveIngredient epSOSActiveIngredient Value Set ID 1.3.6.1.4.1.12559.11.10.1.3.1.42.24 TRANSLATIONS Code System ID Code System Version Concept Code Description (FSN) 2.16.840.1.113883.6.73 2017-01 A ALIMENTARY TRACT AND METABOLISM 2.16.840.1.113883.6.73 2017-01 -

Jp Xvii the Japanese Pharmacopoeia
JP XVII THE JAPANESE PHARMACOPOEIA SEVENTEENTH EDITION Official from April 1, 2016 English Version THE MINISTRY OF HEALTH, LABOUR AND WELFARE Notice: This English Version of the Japanese Pharmacopoeia is published for the convenience of users unfamiliar with the Japanese language. When and if any discrepancy arises between the Japanese original and its English translation, the former is authentic. The Ministry of Health, Labour and Welfare Ministerial Notification No. 64 Pursuant to Paragraph 1, Article 41 of the Law on Securing Quality, Efficacy and Safety of Products including Pharmaceuticals and Medical Devices (Law No. 145, 1960), the Japanese Pharmacopoeia (Ministerial Notification No. 65, 2011), which has been established as follows*, shall be applied on April 1, 2016. However, in the case of drugs which are listed in the Pharmacopoeia (hereinafter referred to as ``previ- ous Pharmacopoeia'') [limited to those listed in the Japanese Pharmacopoeia whose standards are changed in accordance with this notification (hereinafter referred to as ``new Pharmacopoeia'')] and have been approved as of April 1, 2016 as prescribed under Paragraph 1, Article 14 of the same law [including drugs the Minister of Health, Labour and Welfare specifies (the Ministry of Health and Welfare Ministerial Notification No. 104, 1994) as of March 31, 2016 as those exempted from marketing approval pursuant to Paragraph 1, Article 14 of the Same Law (hereinafter referred to as ``drugs exempted from approval'')], the Name and Standards established in the previous Pharmacopoeia (limited to part of the Name and Standards for the drugs concerned) may be accepted to conform to the Name and Standards established in the new Pharmacopoeia before and on September 30, 2017. -

Estonian Statistics on Medicines 2016 1/41
Estonian Statistics on Medicines 2016 ATC code ATC group / Active substance (rout of admin.) Quantity sold Unit DDD Unit DDD/1000/ day A ALIMENTARY TRACT AND METABOLISM 167,8985 A01 STOMATOLOGICAL PREPARATIONS 0,0738 A01A STOMATOLOGICAL PREPARATIONS 0,0738 A01AB Antiinfectives and antiseptics for local oral treatment 0,0738 A01AB09 Miconazole (O) 7088 g 0,2 g 0,0738 A01AB12 Hexetidine (O) 1951200 ml A01AB81 Neomycin+ Benzocaine (dental) 30200 pieces A01AB82 Demeclocycline+ Triamcinolone (dental) 680 g A01AC Corticosteroids for local oral treatment A01AC81 Dexamethasone+ Thymol (dental) 3094 ml A01AD Other agents for local oral treatment A01AD80 Lidocaine+ Cetylpyridinium chloride (gingival) 227150 g A01AD81 Lidocaine+ Cetrimide (O) 30900 g A01AD82 Choline salicylate (O) 864720 pieces A01AD83 Lidocaine+ Chamomille extract (O) 370080 g A01AD90 Lidocaine+ Paraformaldehyde (dental) 405 g A02 DRUGS FOR ACID RELATED DISORDERS 47,1312 A02A ANTACIDS 1,0133 Combinations and complexes of aluminium, calcium and A02AD 1,0133 magnesium compounds A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 811120 pieces 10 pieces 0,1689 A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 3101974 ml 50 ml 0,1292 A02AD83 Calcium carbonate+ Magnesium carbonate (O) 3434232 pieces 10 pieces 0,7152 DRUGS FOR PEPTIC ULCER AND GASTRO- A02B 46,1179 OESOPHAGEAL REFLUX DISEASE (GORD) A02BA H2-receptor antagonists 2,3855 A02BA02 Ranitidine (O) 340327,5 g 0,3 g 2,3624 A02BA02 Ranitidine (P) 3318,25 g 0,3 g 0,0230 A02BC Proton pump inhibitors 43,7324 A02BC01 Omeprazole -

Pharmaceutical Appendix to the Tariff Schedule 2
Harmonized Tariff Schedule of the United States (2007) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2007) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. ABACAVIR 136470-78-5 ACIDUM LIDADRONICUM 63132-38-7 ABAFUNGIN 129639-79-8 ACIDUM SALCAPROZICUM 183990-46-7 ABAMECTIN 65195-55-3 ACIDUM SALCLOBUZICUM 387825-03-8 ABANOQUIL 90402-40-7 ACIFRAN 72420-38-3 ABAPERIDONUM 183849-43-6 ACIPIMOX 51037-30-0 ABARELIX 183552-38-7 ACITAZANOLAST 114607-46-4 ABATACEPTUM 332348-12-6 ACITEMATE 101197-99-3 ABCIXIMAB 143653-53-6 ACITRETIN 55079-83-9 ABECARNIL 111841-85-1 ACIVICIN 42228-92-2 ABETIMUSUM 167362-48-3 ACLANTATE 39633-62-0 ABIRATERONE 154229-19-3 ACLARUBICIN 57576-44-0 ABITESARTAN 137882-98-5 ACLATONIUM NAPADISILATE 55077-30-0 ABLUKAST 96566-25-5 ACODAZOLE 79152-85-5 ABRINEURINUM 178535-93-8 ACOLBIFENUM 182167-02-8 ABUNIDAZOLE 91017-58-2 ACONIAZIDE 13410-86-1 ACADESINE 2627-69-2 ACOTIAMIDUM 185106-16-5 ACAMPROSATE 77337-76-9 -

Acefylline Piperazine/Bambuterol Hydrochloride 1115 1 Mg of Aminophylline
Acefylline Piperazine/Bambuterol Hydrochloride 1115 1 mg of aminophylline. The USP 31 specifies that ami- Oral modified-release preparations are given to children with a Preparations nophylline preparations should be labelled with respect body-weight over 40 kg in the long-term management of chronic Proprietary Preparations (details are given in Part 3) bronchospasm. An initial dose of 225 mg twice daily may be Jpn: Solfa; Neth.: Miraftil; USA: Aphthasol. to their anhydrous theophylline content. As the phar- given if the child has not previously received xanthine prepara- macokinetics of theophylline are affected by a number tions, increased after 1 week to 450 mg twice daily according to of factors including age, smoking, disease, diet, and serum-theophylline concentrations. Different modified-release Arformoterol Tartrate (USAN, rINNM) ⊗ drug interactions, the dose of aminophylline must be preparations are not considered interchangeable. carefully individualised and serum-theophylline con- Aminophylline may also be used in the management of neonatal Arformotérol, Tartrate d’; Arformoteroli Tartras; R,R-Formoterol Tartrate; Tartrato de arformoterol. (-)-N-[2-Hydroxy-5-((1R)-1- centrations monitored (see Uses and Administration of apnoea (see p.1118). Although the injection is unlicensed in the UK in children under 6 months of age, the BNFC recommends hydroxy-2-{[(1R)-2-(4-methoxyphenyl)-1-methylethyl]ami- Theophylline, p.1146). an initial dose of 6 mg/kg by intravenous injection over 20 min- no}ethyl)phenyl]formamide hydrogen (2R,3R)-2,3-dihydroxybu- In the management of acute severe bronchospasm, utes. This is followed by 2.5 mg/kg every 12 hours, increased if tanedioate. aminophylline may be given intravenously by slow in- necessary to 3.5 mg/kg every 12 hours. -
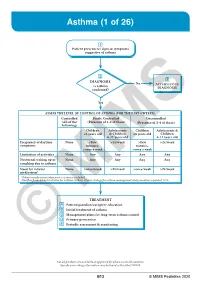
Asthma (1 of 26)
Asthma (1 of 26) 1 Patient presents w/ signs & symptoms suggestive of asthma 2 3 DIAGNOSIS No ALTERNATIVE Is asthma DIAGNOSIS confi rmed? Yes ASSESS THE LEVEL OF CONTROL OF ASTHMA FOR THE PAST 4 WEEKS Controlled Partly Controlled Uncontrolled (All of the (Presence of 1-2 of these) (Presence of 3-4 of these) following) Children Adolescents Children Adolescents & ≤5 years old & Children ≤5 years old Children 6-11 years old 6-11 years old Frequency of daytime None >Few >2x/week >Few >2x/week symptoms minutes, minutes, >once a week >once a week Limitation of activities None Any Any Any Any Nocturnal waking up or None Any Any Any Any coughing due to asthma Need for reliever None >once/week >2x/week >once/week >2x/week medication* *Reliever medications taken prior to exercise excluded. Modified from: Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention: Updated 2020. TREATMENT A Patient/guardian/caregiver education B Initial treatment of asthma C Management plans for long-term asthma control D Primary prevention E © Periodic assessmentMIMS & monitoring Not all products are available or approved for above use in all countries. Specifi c prescribing information may be found in the latest MIMS. B13 © MIMS Pediatrics 2020 Asthma (2 of 26) 1 ASTHMA • A heterogeneous disease w/ chronic infl ammatory disorder of the airways • e most common chronic disease in pediatric age groups that causes signifi cant morbidity • Characterized by history of respiratory symptoms eg wheeze, shortness of breath, chest tightness & cough