Identification and Caste-Dependent Expression Patterns of DNA
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Anthidium Manicatum, an Invasive Bee, Excludes a Native Bumble Bee, Bombus Impatiens, from floral Resources
Biol Invasions https://doi.org/10.1007/s10530-018-1889-7 (0123456789().,-volV)(0123456789().,-volV) ORIGINAL PAPER Anthidium manicatum, an invasive bee, excludes a native bumble bee, Bombus impatiens, from floral resources Kelsey K. Graham . Katherine Eaton . Isabel Obrien . Philip T. Starks Received: 15 April 2018 / Accepted: 21 November 2018 Ó Springer Nature Switzerland AG 2018 Abstract Anthidium manicatum is an invasive pol- response to A. manicatum presence. We found that B. linator reaching widespread distribution in North impatiens avoided foraging near A. manicatum in both America. Male A. manicatum aggressively defend years; but despite this resource exclusion, we found no floral territories, attacking heterospecific pollinators. evidence of fitness consequences for B. impatiens. Female A. manicatum are generalists, visiting many of These results suggest A. manicatum pose as significant the same plants as native pollinators. Because of A. resource competitors, but that B. impatiens are likely manicatum’s rapid range expansion, the territorial able to compensate for this resource loss by finding behavior of males, and the potential for female A. available resources elsewhere. manicatum to be significant resource competitors, invasive A. manicatum have been prioritized as a Keywords Exotic species Á Resource competition Á species of interest for impact assessment. But despite Interspecific competition Á Foraging behavior Á concerns, there have been no empirical studies inves- Pollination tigating the impact of A. manicatum on North Amer- ican pollinators. Therefore, across a two-year study, we monitored foraging behavior and fitness of the common eastern bumble bee (Bombus impatiens) in Introduction With increasing movement of goods and people Electronic supplementary material The online version of around the world, introduction of exotic species is this article (https://doi.org/10.1007/s10530-018-1889-7) con- increasing at an unprecedented rate (Ricciardi et al. -

THE HUMBLE-BEE MACMILLAN and CO., Limited LONDON BOMBAY CALCUTTA MELBOURNE the MACMILLAN COMPANY NEW YORK BOSTON CHICAGO DALLAS SAN FRANCISCO the MACMILLAN CO
THE HUMBLE-BEE MACMILLAN AND CO., Limited LONDON BOMBAY CALCUTTA MELBOURNE THE MACMILLAN COMPANY NEW YORK BOSTON CHICAGO DALLAS SAN FRANCISCO THE MACMILLAN CO. OF CANADA, Ltd. TORONTO A PET QUEEN OF BOMBUS TERRESTRIS INCUBATING HER BROOD. (See page 139.) THE HUMBLE-BEE ITS LIFE-HISTORY AND HOW TO DOMESTICATE IT WITH DESCRIPTIONS OF ALL THE BRITISH SPECIES OF BOMBUS AND PSITHTRUS BY \ ; Ff W. U SLADEN FELLOW OF THE ENTOMOLOGICAL SOCIETY OF LONDON AUTHOR OF 'QUEEN-REARING IN ENGLAND ' ILLUSTRATED WITH PHOTOGRAPHS AND DRAWINGS BY THE AUTHOR AND FIVE COLOURED PLATES PHOTOGRAPHED DIRECT FROM NA TURE MACMILLAN AND CO., LIMITED ST. MARTIN'S STREET, LONDON 1912 COPYRIGHT Printed in ENGLAND. PREFACE The title, scheme, and some of the contents of this book are borrowed from a little treatise printed on a stencil copying apparatus in August 1892. The boyish effort brought me several naturalist friends who encouraged me to pursue further the study of these intelligent and useful insects. ..Of these friends, I feel especially indebted to the late Edward Saunders, F.R.S., author of The Hymen- optera Aculeata of the British Islands, and to the late Mrs. Brightwen, the gentle writer of Wild Nattcre Won by Kindness, and other charming studies of pet animals. The general outline of the life-history of the humble-bee is, of course, well known, but few observers have taken the trouble to investigate the details. Even Hoffer's extensive monograph, Die Htimmeln Steiermarks, published in 1882 and 1883, makes no mention of many remarkable can particulars that I have witnessed, and there be no doubt that further investigations will reveal more. -

Bombus Terrestris L
Apidologie 39 (2008) 419–427 Available online at: c INRA/DIB-AGIB/ EDP Sciences, 2008 www.apidologie.org DOI: 10.1051/apido:2008020 Original article Foraging distance in Bombus terrestris L. (Hymenoptera: Apidae)* Stephan Wolf, Robin F.A. Moritz Institut für Biologie / Institutsbereich Zoologie, Martin-Luther-Universität Halle-Wittenberg, Germany Received 11 October 2007 – Revised 7 February 2008 – Accepted 25 February 2008 Abstract – A major determinant of bumblebees pollination efficiency is the distance of pollen dispersal, which depends on the foraging distance of workers. We employ a transect setting, controlling for both forage and nest location, to assess the foraging distance of Bombus terrestris workers and the influence of environmental factors on foraging frequency over distance. The mean foraging distance of B. terrestris workers was 267.2 m ± 180.3 m (max. 800 m). Nearly 40% of the workers foraged within 100 m around the nest. B. terrestris workers have thus rather moderate foraging ranges if rewarding forage is available within vicinity of the nests. We found the spatial distribution and the quality of forage plots to be the major determinants for the bees foraging decision-making, explaining over 80% of the foraging frequency. This low foraging range has implications for using B. terrestris colonies as pollinators in agriculture. Bumblebee / foraging / pollination / decision-making 1. INTRODUCTION efficiency (Gauld et al., 1990; Westerkamp, 1991; Wilson and Thomson, 1991; Goulson, Pollen dispersal through animal pollinators 2003). This is partly due to the more robust is essential for plant reproduction. The effi- handling of flowers by bumblebees and their ciency of pollinators depends on various fac- ability of buzz-pollination (e.g. -
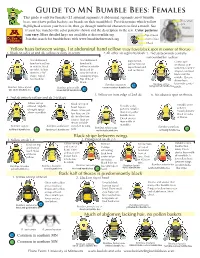
Guide to MN Bumble Bees: Females
Guide to MN Bumble Bees: Females This guide is only for females (12 antennal segments, 6 abdominal segments, most bumble Three small bees, most have pollen baskets, no beards on their mandibles). First determine which yellow eyes highlighted section your bee is in, then go through numbered characters to find a match. See if your bee matches the color patterns shown and the description in the text. Color patterns ® can vary. More detailed keys are available at discoverlife.org. Top of head Bee Front of face Squad Join the search for bumble bees with www.bumbleebeewatch.org Cheek Yellow hairs between wings, 1st abdominal band yellow (may have black spot in center of thorax) 1. Black on sides of 2nd ab, yellow or rusty in center 2.All other ab segments black 3. 2nd ab brownish centrally surrounded by yellow 2nd abdominal 2nd abdominal Light lemon Center spot band with yellow band with yellow hairs on on thorax with in middle, black yellow in middle top of head and sometimes faint V on sides. Yellow bordered by and on thorax. shaped extension often in a “W” rusty brown in a back from the shape. Top of swooping shape. middle. Queens head yellow. Top of head do not have black. Bombus impatiens Bombus affinis brownish central rusty patched bumble bee Bombus bimaculatus Bombus griseocollis common eastern bumble bee C patch. two-spotted bumble bee C brown-belted bumble bee C 5. Yellow on front edge of 2nd ab 6. No obvious spot on thorax. 4. 2nd ab entirely yellow and ab 3-6 black Yellow on top Black on top of Variable color of head. -

Bumble Bee Abundance in New York City Community Gardens: Implications for Urban Agriculture
Matteson and Langellotto: URBAN BUMBLE BEE ABUNDANCE Cities and the Environment 2009 Volume 2, Issue 1 Article 5 Bumble Bee Abundance in New York City Community Gardens: Implications for Urban Agriculture Kevin C. Matteson and Gail A. Langellotto Abstract A variety of crops are grown in New York City community gardens. Although the production of many crops benefits from pollination by bees, little is known about bee abundance in urban community gardens or which crops are specifically dependent on bee pollination. In 2005, we compiled a list of crop plants grown within 19 community gardens in New York City and classified these plants according to their dependence on bee pollination. In addition, using mark-recapture methods, we estimated the abundance of a potentially important pollinator within New York City urban gardens, the common eastern bumble bee (Bombus impatiens). This species is currently recognized as a valuable commercial pollinator of greenhouse crops. However, wild populations of B. impatiens are abundant throughout its range, including in New York City community gardens, where it is the most abundant native bee species present and where it has been observed visiting a variety of crop flowers. We conservatively counted 25 species of crop plants in 19 surveyed gardens. The literature suggests that 92% of these crops are dependent, to some degree, on bee pollination in order to set fruit or seed. Bombus impatiens workers were observed visiting flowers of 78% of these pollination-dependent crops. Estimates of the number of B. impatiens workers visiting individual gardens during the study period ranged from 3 to 15 bees per 100 m2 of total garden area and 6 to 29 bees per 100 m2 of garden floral area. -

Conserving Missouri's Wild and Managed Pollinators
Conserving Missouri’s Wild and Managed Pollinators At the heart of the pollination issue lies our bounty of foods such as peaches, Go to the bee, thou poet: strawberries, squash and apples. These and other foods requiring pollination have consider her ways and be wise. been staples in the human diet for centuries, and their pollinators have been highly — George Bernard Shaw revered since ancient times. From Egyptian hieroglyphics and Native American cave paintings to Greek mythology and English poetry, bees and butterflies have been a source of fascination and awe for millennia (Figure 1). Yet, over the past century, pollinator numbers have suffered declines. During this time, global development, a booming human population, and industrial agriculture brought about drastic landscape changes. These changes have resulted in greater losses of forage and nesting resources for pollinators than ever before seen. The ecosystem service of pollination, once taken for granted, is now potentially threatened as many pollinator species face declines in Missouri, the United States and many regions around the world. Pollinators are critically important for natural ecosystems and crop production. Through pollination, they perform roles essential to human welfare. Heightened public awareness of their services and of their declines over the past two decades has prompted action, but much remains to be done. This publication introduces issues regarding the conservation of pollinators in Missouri. It explores why pollinators are crucial, what major threats confront them, what conservation steps are being taken, and how you can help. It highlights bees over other pollinators as bees are the most important for both agricultural and natural pollination in Missouri. -

Evidence for and Against Deformed Wing Virus Spillover from Honey Bees to Bumble Bees: a Reverse Genetic Analysis Olesya N
www.nature.com/scientificreports OPEN Evidence for and against deformed wing virus spillover from honey bees to bumble bees: a reverse genetic analysis Olesya N. Gusachenko1*, Luke Woodford1, Katharin Balbirnie‑Cumming1, Eugene V. Ryabov2 & David J. Evans1* Deformed wing virus (DWV) is a persistent pathogen of European honey bees and the major contributor to overwintering colony losses. The prevalence of DWV in honey bees has led to signifcant concerns about spillover of the virus to other pollinating species. Bumble bees are both a major group of wild and commercially‑reared pollinators. Several studies have reported pathogen spillover of DWV from honey bees to bumble bees, but evidence of a sustained viral infection characterized by virus replication and accumulation has yet to be demonstrated. Here we investigate the infectivity and transmission of DWV in bumble bees using the buf-tailed bumble bee Bombus terrestris as a model. We apply a reverse genetics approach combined with controlled laboratory conditions to detect and monitor DWV infection. A novel reverse genetics system for three representative DWV variants, including the two master variants of DWV—type A and B—was used. Our results directly confrm DWV replication in bumble bees but also demonstrate striking resistance to infection by certain transmission routes. Bumble bees may support DWV replication but it is not clear how infection could occur under natural environmental conditions. Deformed wing virus (DWV) is a widely established pathogen of the European honey bee, Apis mellifera. In synergistic action with its vector—the parasitic mite Varroa destructor—it has had a devastating impact on the health of honey bee colonies globally1,2. -

Bombus Terrestris) Colonies
veterinary sciences Article Replicative Deformed Wing Virus Found in the Head of Adults from Symptomatic Commercial Bumblebee (Bombus terrestris) Colonies Giovanni Cilia , Laura Zavatta, Rosa Ranalli, Antonio Nanetti * and Laura Bortolotti CREA Research Centre for Agriculture and Environment, Via di Saliceto 80, 40128 Bologna, Italy; [email protected] (G.C.); [email protected] (L.Z.); [email protected] (R.R.); [email protected] (L.B.) * Correspondence: [email protected] Abstract: The deformed wing virus (DWV) is one of the most common honey bee pathogens. The virus may also be detected in other insect species, including Bombus terrestris adults from wild and managed colonies. In this study, individuals of all stages, castes, and sexes were sampled from three commercial colonies exhibiting the presence of deformed workers and analysed for the presence of DWV. Adults (deformed individuals, gynes, workers, males) had their head exscinded from the rest of the body and the two parts were analysed separately by RT-PCR. Juvenile stages (pupae, larvae, and eggs) were analysed undissected. All individuals tested positive for replicative DWV, but deformed adults showed a higher number of copies compared to asymptomatic individuals. Moreover, they showed viral infection in their heads. Sequence analysis indicated that the obtained DWV amplicons belonged to a strain isolated in the United Kingdom. Further studies are needed to Citation: Cilia, G.; Zavatta, L.; characterize the specific DWV target organs in the bumblebees. The result of this study indicates the Ranalli, R.; Nanetti, A.; Bortolotti, L. evidence of DWV infection in B. -

Complementary Symbiont Contributions to Plant Decomposition in a Fungus-Farming Termite
Complementary symbiont contributions to plant decomposition in a fungus-farming termite Michael Poulsena,1,2, Haofu Hub,1, Cai Lib,c, Zhensheng Chenb, Luohao Xub, Saria Otania, Sanne Nygaarda, Tania Nobred,3, Sylvia Klaubaufe, Philipp M. Schindlerf, Frank Hauserg, Hailin Panb, Zhikai Yangb, Anton S. M. Sonnenbergh, Z. Wilhelm de Beeri, Yong Zhangb, Michael J. Wingfieldi, Cornelis J. P. Grimmelikhuijzeng, Ronald P. de Vriese, Judith Korbf,4, Duur K. Aanend, Jun Wangb,j, Jacobus J. Boomsmaa, and Guojie Zhanga,b,2 aCentre for Social Evolution, Department of Biology, University of Copenhagen, DK-2100 Copenhagen, Denmark; bChina National Genebank, BGI-Shenzen, Shenzhen 518083, China; cCentre for GeoGenetics, Natural History Museum of Denmark, University of Copenhagen, DK-1350 Copenhagen, Denmark; dLaboratory of Genetics, Wageningen University, 6708 PB, Wageningen, The Netherlands; eFungal Biodiversity Centre, Centraalbureau voor Schimmelcultures, Royal Netherlands Academy of Arts and Sciences, NL-3584 CT, Utrecht, The Netherlands; fBehavioral Biology, Fachbereich Biology/Chemistry, University of Osnabrück, D-49076 Osnabrück, Germany; gCenter for Functional and Comparative Insect Genomics, Department of Biology, University of Copenhagen, DK-2100 Copenhagen, Denmark; hDepartment of Plant Breeding, Wageningen University and Research Centre, NL-6708 PB, Wageningen, The Netherlands; iDepartment of Microbiology, Forestry and Agricultural Biotechnology Institute, University of Pretoria, Pretoria SA-0083, South Africa; and jDepartment of Biology, University of Copenhagen, DK-2100 Copenhagen, Denmark Edited by Ian T. Baldwin, Max Planck Institute for Chemical Ecology, Jena, Germany, and approved August 15, 2014 (received for review October 24, 2013) Termites normally rely on gut symbionts to decompose organic levels-of-selection conflicts that need to be regulated (12). -
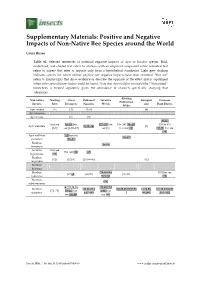
Positive and Negative Impacts of Non-Native Bee Species Around the World
Supplementary Materials: Positive and Negative Impacts of Non-Native Bee Species around the World Laura Russo Table S1. Selected references of potential negative impacts of Apis or Bombus species. Bold, underlined, and shaded text refers to citations with an empirical component while unbolded text refers to papers that refer to impacts only from a hypothetical standpoint. Light grey shading indicates species for which neither positive nor negative impacts have been recorded. “But see” refers to manuscripts that show evidence or describe the opposite of the effect and is capitalized when only contradictory studies could be found. Note that Apis mellifera scutellata (the “Africanized” honeybee), is treated separately given the abundance of research specifically studying that subspecies. Altering Non-native Nesting Floral Pathoens/ Invasive Introgres Decrease Pollination Species Sites Resources Parasites Weeds sion Plant Fitness Webs Apis cerana [1] [2] [1–3] [4] Apis dorsata Apis florea [5] [5] [37,45] But see [8–19] but [27–35] but [36–38] [39–43] [38,46,47] Apis mellifera [9,23–26] [4] [6,7] see [6,20–22] see [6] but see [44] [48,49] but see [50] Apis mellifera [51] but see [55–57] scutellata [52–54] Bombus [58,59] hortorum Bombus But see But see [60] [61] hypnorum [60] Bombus [62] [62,63] [26,64–66] [62] impatiens Bombus lucorum Bombus [28,58,59,6 [39] but see [67,68] [69,70] [36,39] ruderatus 9,71,72] [73] Bombus [59] subterraneous [67,70,74,75, [29,58,72,9 Bombus [25,26,70,7 [38,39,68,81,97,98 [4,76,88, [47,76,49,86,97 [74–76] 77–84] but 1–95] but terrestris 6,87–90] ] 99,100] ,101–103] see [85,86] see [96] Insects 2016, 7, 69; doi:10.3390/insects7040069 www.mdpi.com/journal/insects Insects 2016, 7, 69 S2 of S8 Table S2. -

An Abstract of the Thesis Of
AN ABSTRACT OF THE THESIS OF Sarah A. Maxfield-Taylor for the degree of Master of Science in Entomology presented on March 26, 2014. Title: Natural Enemies of Native Bumble Bees (Hymenoptera: Apidae) in Western Oregon Abstract approved: _____________________________________________ Sujaya U. Rao Bumble bees (Hymenoptera: Apidae) are important native pollinators in wild and agricultural systems, and are one of the few groups of native bees commercially bred for use in the pollination of a range of crops. In recent years, declines in bumble bees have been reported globally. One factor implicated in these declines, believed to affect bumble bee colonies in the wild and during rearing, is natural enemies. A diversity of fungi, protozoa, nematodes, and parasitoids has been reported to affect bumble bees, to varying extents, in different parts of the world. In contrast to reports of decline elsewhere, bumble bees have been thriving in Oregon on the West Coast of the U.S.A.. In particular, the agriculturally rich Willamette Valley in the western part of the state appears to be fostering several species. Little is known, however, about the natural enemies of bumble bees in this region. The objectives of this thesis were to: (1) identify pathogens and parasites in (a) bumble bees from the wild, and (b) bumble bees reared in captivity and (2) examine the effects of disease on bee hosts. Bumble bee queens and workers were collected from diverse locations in the Willamette Valley, in spring and summer. Bombus mixtus, Bombus nevadensis, and Bombus vosnesenskii collected from the wild were dissected and examined for pathogens and parasites, and these organisms were identified using morphological and molecular characteristics. -

Bombus Impatiens, Common Eastern Bumblebee
Bombus impatiens, Common Eastern Bumble Bee (Hymenoptera: Apidae) Christopher J. Fellows, Forest Huval, T.E. Reagan and Chris Carlton Description The common eastern bumble bee, Bombus impatiens, is an important native pollinator found in the eastern United States and southern Canada. Adult bees are covered with short, even hairs across their bodies. They are mostly black with a yellow thorax (middle section) and an additional yellow stripe at the base of the abdomen. As with other social insects, the different forms, or castes, of B. impatiens vary in size. Worker bumble bees measure from 1/3 to 2/3 of an inch in length (8.5-16 mm), while drones measure ½ to ¾ of an inch (12 to 18 mm). Queen bumble bees are the largest members of the three castes, measuring between 1/3 of an inch to 1 inch (17 and 23 mm) in length. Larvae are rarely seen and are enclosed with the nest brood cells for the duration of their A common eastern bumble bee resting on a flower. Note development. They are pale, legless grubs around 1 inch the yellow patch of hairs on the forward portion of the thorax (David Cappaert, Bugwood.org). (25 mm) in length. At least six additional species of bumble bees have been construction. Within the hollow fiber ball, the queen documented in Louisiana, with another two species possibly deposits a lump of nectar-moistened pollen. The queens present but not confirmed. Identifications are mainly based also construct honey pots near the nest entrance using on the patterns of coloration of the body hairs, but these the secretions of her wax glands.