Mass Flowering Crops As a Conservation Resource for Wild Pollinators (Hymenoptera: Apoidea)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Taxon Order Family Scientific Name Common Name Non-Native No. of Individuals/Abundance Notes Bees Hymenoptera Andrenidae Calliop
Taxon Order Family Scientific Name Common Name Non-native No. of individuals/abundance Notes Bees Hymenoptera Andrenidae Calliopsis andreniformis Mining bee 5 Bees Hymenoptera Apidae Apis millifera European honey bee X 20 Bees Hymenoptera Apidae Bombus griseocollis Brown belted bumble bee 1 Bees Hymenoptera Apidae Bombus impatiens Common eastern bumble bee 12 Bees Hymenoptera Apidae Ceratina calcarata Small carpenter bee 9 Bees Hymenoptera Apidae Ceratina mikmaqi Small carpenter bee 4 Bees Hymenoptera Apidae Ceratina strenua Small carpenter bee 10 Bees Hymenoptera Apidae Melissodes druriella Small carpenter bee 6 Bees Hymenoptera Apidae Xylocopa virginica Eastern carpenter bee 1 Bees Hymenoptera Colletidae Hylaeus affinis masked face bee 6 Bees Hymenoptera Colletidae Hylaeus mesillae masked face bee 3 Bees Hymenoptera Colletidae Hylaeus modestus masked face bee 2 Bees Hymenoptera Halictidae Agapostemon virescens Sweat bee 7 Bees Hymenoptera Halictidae Augochlora pura Sweat bee 1 Bees Hymenoptera Halictidae Augochloropsis metallica metallica Sweat bee 2 Bees Hymenoptera Halictidae Halictus confusus Sweat bee 7 Bees Hymenoptera Halictidae Halictus ligatus Sweat bee 2 Bees Hymenoptera Halictidae Lasioglossum anomalum Sweat bee 1 Bees Hymenoptera Halictidae Lasioglossum ellissiae Sweat bee 1 Bees Hymenoptera Halictidae Lasioglossum laevissimum Sweat bee 1 Bees Hymenoptera Halictidae Lasioglossum platyparium Cuckoo sweat bee 1 Bees Hymenoptera Halictidae Lasioglossum versatum Sweat bee 6 Beetles Coleoptera Carabidae Agonum sp. A ground beetle -

Anthidium Manicatum, an Invasive Bee, Excludes a Native Bumble Bee, Bombus Impatiens, from floral Resources
Biol Invasions https://doi.org/10.1007/s10530-018-1889-7 (0123456789().,-volV)(0123456789().,-volV) ORIGINAL PAPER Anthidium manicatum, an invasive bee, excludes a native bumble bee, Bombus impatiens, from floral resources Kelsey K. Graham . Katherine Eaton . Isabel Obrien . Philip T. Starks Received: 15 April 2018 / Accepted: 21 November 2018 Ó Springer Nature Switzerland AG 2018 Abstract Anthidium manicatum is an invasive pol- response to A. manicatum presence. We found that B. linator reaching widespread distribution in North impatiens avoided foraging near A. manicatum in both America. Male A. manicatum aggressively defend years; but despite this resource exclusion, we found no floral territories, attacking heterospecific pollinators. evidence of fitness consequences for B. impatiens. Female A. manicatum are generalists, visiting many of These results suggest A. manicatum pose as significant the same plants as native pollinators. Because of A. resource competitors, but that B. impatiens are likely manicatum’s rapid range expansion, the territorial able to compensate for this resource loss by finding behavior of males, and the potential for female A. available resources elsewhere. manicatum to be significant resource competitors, invasive A. manicatum have been prioritized as a Keywords Exotic species Á Resource competition Á species of interest for impact assessment. But despite Interspecific competition Á Foraging behavior Á concerns, there have been no empirical studies inves- Pollination tigating the impact of A. manicatum on North Amer- ican pollinators. Therefore, across a two-year study, we monitored foraging behavior and fitness of the common eastern bumble bee (Bombus impatiens) in Introduction With increasing movement of goods and people Electronic supplementary material The online version of around the world, introduction of exotic species is this article (https://doi.org/10.1007/s10530-018-1889-7) con- increasing at an unprecedented rate (Ricciardi et al. -
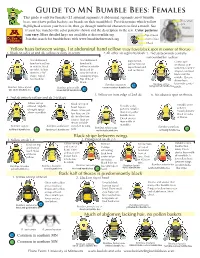
Guide to MN Bumble Bees: Females
Guide to MN Bumble Bees: Females This guide is only for females (12 antennal segments, 6 abdominal segments, most bumble Three small bees, most have pollen baskets, no beards on their mandibles). First determine which yellow eyes highlighted section your bee is in, then go through numbered characters to find a match. See if your bee matches the color patterns shown and the description in the text. Color patterns ® can vary. More detailed keys are available at discoverlife.org. Top of head Bee Front of face Squad Join the search for bumble bees with www.bumbleebeewatch.org Cheek Yellow hairs between wings, 1st abdominal band yellow (may have black spot in center of thorax) 1. Black on sides of 2nd ab, yellow or rusty in center 2.All other ab segments black 3. 2nd ab brownish centrally surrounded by yellow 2nd abdominal 2nd abdominal Light lemon Center spot band with yellow band with yellow hairs on on thorax with in middle, black yellow in middle top of head and sometimes faint V on sides. Yellow bordered by and on thorax. shaped extension often in a “W” rusty brown in a back from the shape. Top of swooping shape. middle. Queens head yellow. Top of head do not have black. Bombus impatiens Bombus affinis brownish central rusty patched bumble bee Bombus bimaculatus Bombus griseocollis common eastern bumble bee C patch. two-spotted bumble bee C brown-belted bumble bee C 5. Yellow on front edge of 2nd ab 6. No obvious spot on thorax. 4. 2nd ab entirely yellow and ab 3-6 black Yellow on top Black on top of Variable color of head. -

Wedge-Shaped Beetles (Suggested Common Name) Ripiphorus Spp. (Insecta: Coleoptera: Ripiphoridae)1 David Owens, Ashley N
EENY613 Wedge-Shaped Beetles (suggested common name) Ripiphorus spp. (Insecta: Coleoptera: Ripiphoridae)1 David Owens, Ashley N. Mortensen, Jeanette Klopchin, William Kern, and Jamie D. Ellis2 Introduction Ripiphoridae are a family of unusual parasitic beetles that are thought to be related to tumbling flower beetles (Coleoptera: Mordellidae) and blister beetles (Coleoptera: Meloidae). There is disagreement over the spelling of the family (Ripiphoridae) and genus (Ripiphorus) names. Here we use the original spelling that starts with only the letter Figure 1. Adult specimens of the two genera of Ripiphoridae. A) “R”; however, an initial “Rh” has also been used in the Macrosiagon Hentz, and B) Ripiphorus Bosc. scientific community (Rhipiphoridae and Rhipiphorus). Credits: Allen M. Boatman There are an estimated 35 nearctic species of Ripiphorus, Generally, the biology of the family Ripiphoridae is poorly two of which have been collected in Florida: Ripiphorus known. Ripiphorids parasitize bees and wasps (Hymenop- schwarzi LeConte (Figure 2A) and Ripiphorus fasciatus Say tera), roaches (Blattodea), and wood-boring beetles (Co- (Figure 2B). Due to limited information for both of these leoptera). However, the specific hosts for many ripiphorid species, the information presented below is characteristic species are unknown. Furthermore, only one sex (either of the genus Ripiphorus. Information specific to Ripiphorus male or female) has been described for several species, and fasciatus and Ripiphorus schwarzi is presented where the males and females of some species look different. detailed information is available. Two genera of Ripiphoridae infest hymenopteran (bee and wasp) nests: Macrosiagon Hentz (Figure 1A) and Ripiphorus Bosc (formerly Myodites Latreille) (Figure 1B). Species of Macrosiagon are parasites of a variety of hymenopteran families including: Halictidae, Vespidae, Tiphiidae, Apidae, Pompilidae, Crabronidae, and Sphecidae. -

Growing a Wild NYC: a K-5 Urban Pollinator Curriculum Was Made Possible Through the Generous Support of Our Funders
A K-5 URBAN POLLINATOR CURRICULUM Growing a Wild NYC LESSON 1: HABITAT HUNT The National Wildlife Federation Uniting all Americans to ensure wildlife thrive in a rapidly changing world Through educational programs focused on conservation and environmental knowledge, the National Wildlife Federation provides ways to create a lasting base of environmental literacy, stewardship, and problem-solving skills for today’s youth. Growing a Wild NYC: A K-5 Urban Pollinator Curriculum was made possible through the generous support of our funders: The Seth Sprague Educational and Charitable Foundation is a private foundation that supports the arts, housing, basic needs, the environment, and education including professional development and school-day enrichment programs operating in public schools. The Office of the New York State Attorney General and the New York State Department of Environmental Conservation through the Greenpoint Community Environmental Fund. Written by Nina Salzman. Edited by Sarah Ward and Emily Fano. Designed by Leslie Kameny, Kameny Design. © 2020 National Wildlife Federation. Permission granted for non-commercial educational uses only. All rights reserved. September - January Lesson 1: Habitat Hunt Page 8 Lesson 2: What is a Pollinator? Page 20 Lesson 3: What is Pollination? Page 30 Lesson 4: Why Pollinators? Page 39 Lesson 5: Bee Survey Page 45 Lesson 6: Monarch Life Cycle Page 55 Lesson 7: Plants for Pollinators Page 67 Lesson 8: Flower to Seed Page 76 Lesson 9: Winter Survival Page 85 Lesson 10: Bee Homes Page 97 February -

Bumble Bee Abundance in New York City Community Gardens: Implications for Urban Agriculture
Matteson and Langellotto: URBAN BUMBLE BEE ABUNDANCE Cities and the Environment 2009 Volume 2, Issue 1 Article 5 Bumble Bee Abundance in New York City Community Gardens: Implications for Urban Agriculture Kevin C. Matteson and Gail A. Langellotto Abstract A variety of crops are grown in New York City community gardens. Although the production of many crops benefits from pollination by bees, little is known about bee abundance in urban community gardens or which crops are specifically dependent on bee pollination. In 2005, we compiled a list of crop plants grown within 19 community gardens in New York City and classified these plants according to their dependence on bee pollination. In addition, using mark-recapture methods, we estimated the abundance of a potentially important pollinator within New York City urban gardens, the common eastern bumble bee (Bombus impatiens). This species is currently recognized as a valuable commercial pollinator of greenhouse crops. However, wild populations of B. impatiens are abundant throughout its range, including in New York City community gardens, where it is the most abundant native bee species present and where it has been observed visiting a variety of crop flowers. We conservatively counted 25 species of crop plants in 19 surveyed gardens. The literature suggests that 92% of these crops are dependent, to some degree, on bee pollination in order to set fruit or seed. Bombus impatiens workers were observed visiting flowers of 78% of these pollination-dependent crops. Estimates of the number of B. impatiens workers visiting individual gardens during the study period ranged from 3 to 15 bees per 100 m2 of total garden area and 6 to 29 bees per 100 m2 of garden floral area. -

Conserving Missouri's Wild and Managed Pollinators
Conserving Missouri’s Wild and Managed Pollinators At the heart of the pollination issue lies our bounty of foods such as peaches, Go to the bee, thou poet: strawberries, squash and apples. These and other foods requiring pollination have consider her ways and be wise. been staples in the human diet for centuries, and their pollinators have been highly — George Bernard Shaw revered since ancient times. From Egyptian hieroglyphics and Native American cave paintings to Greek mythology and English poetry, bees and butterflies have been a source of fascination and awe for millennia (Figure 1). Yet, over the past century, pollinator numbers have suffered declines. During this time, global development, a booming human population, and industrial agriculture brought about drastic landscape changes. These changes have resulted in greater losses of forage and nesting resources for pollinators than ever before seen. The ecosystem service of pollination, once taken for granted, is now potentially threatened as many pollinator species face declines in Missouri, the United States and many regions around the world. Pollinators are critically important for natural ecosystems and crop production. Through pollination, they perform roles essential to human welfare. Heightened public awareness of their services and of their declines over the past two decades has prompted action, but much remains to be done. This publication introduces issues regarding the conservation of pollinators in Missouri. It explores why pollinators are crucial, what major threats confront them, what conservation steps are being taken, and how you can help. It highlights bees over other pollinators as bees are the most important for both agricultural and natural pollination in Missouri. -

The Effects of Repeated Prescribed Fire and Thinning on Bees, Wasps, and Other Flower Visitors in the Understory and Midstory of a Temperate Forest in North Carolina
For. Sci. 00(00):1–8 APPLIED RESEARCH doi: 10.1093/forsci/fxx008 Copyright © 2018 Society of American Foresters forest management The Effects of Repeated Prescribed Fire and Thinning on Bees, Wasps, and Other Flower Visitors in the Understory and Midstory of a Temperate Forest in North Carolina Joshua W. Campbell1, Patrick A. Vigueira2, Cynthia C. Viguiera2, and Cathryn H. Greenberg3 We investigated the effects of repeated prescribed fire, mechanical thinning, and combinations of fire and mechanical thinning on pollinators and flower visitors within the herbaceous understory and midstory of a temperate forest in North Carolina. Using colored pan traps, we sampled flower visitors during the plant growing season between 2014 and 2016. We captured 5,520 flower visitors that were dominated by halictid bees and vespid wasps. Twenty genera of bees representing at least 30 species were captured within our experimental plots. Within the forest understory, we found higher abundances and diversities of bees and other flower visitors within plots that had been treated with prescribed fire or a combination of mechanical thinning and prescribed fire compared to control plots. Within our midstory samples, we found that forest manage- ment practices did not affect the abundance of any common flower visitor species/family. However,Augochlora pura and Vespula spp. were more abundant in the midstory compared to the forest understory. Overall, our study demonstrates that repeated applications of prescribed fire maintained elevated abundances and diversity of bees and other flower-visiting insects compared to untreated plots, likely due to increased herbaceous plant diversity and enhanced quality of nesting habitat within the understory. -

Pollinators in Forests – an Annotated Bibliography
Pollinators in Forests – An Annotated Bibliography This annotated bibliography was compiled while researching pollinators in the woods and other related topics. Some text was copied directly from the source when it was appropriately concise. The document is broken into 9 categories: Forest Pollinator Biology, Phenology, Habitat; Forest Pollinators and Woody Material; Forest Management and Pollinators; Forest Pollinators and Agriculture; Forest Pollinators and Habitat Fragments; Forest Pollinators and Adjacent Land Use; Forest Pollinators and Invasive Plants; Pollinator Research Methodology; and Miscellaneous, for papers that do not fit a specific category but that contain relevant information. Forest Pollinator Biology, Phenology, Habitat 1. Adamson, Nancy Lee, et al. “Pollinator Plants: Great Lakes Region,” Xerces Society for Invertebrate Conservation, 2017. Includes a list of plants beneficial to pollinators native to the Great Lakes region, which includes land in Ontario, Minnesota, Wisconsin, Ohio, Michigan, Pennsylvania, and New York. 2. Adamson, Nancy Lee, et al. “Pollinator Plants: Northeast Region,” Xerces Society for Invertebrate Conservation, 2015. Includes a list of plants beneficial to pollinators native to the Northeast Region, which encompasses southern Quebec, New Brunswick, Nova Scotia, the New England states, and eastern New York. 3. Black, Scott Hoffman, et al. “Pollinators in Natural Areas: A Primer on Habitat Management,” Xerces Society for Invertebrate Conservation. By aiding in wildland food production, helping with nutrient cycling, and as direct prey, pollinators are important in wildlife food webs. Summerville and Crist (2002) found that forest moths play important functional roles as selective herbivores, pollinators, detritivores, and prey for migratory songbirds. Belfrage et al. (2005) demonstrated that butterfly diversity was a good predictor of bird abundance and diversity, apparently due to a shared requirement for a complex plant community. -

Native Bee Benefits
Bryn Mawr College and Rutgers University May 2009 Native Bee Benefits How to increase native bee pollination on your farm in several simple steps For Pennsylvania and New Jersey Farmers In this pamphlet, Why are native bees important? Insect pollination services are a highly important you can find out… agricultural input. Two-thirds of crop varieties require animal pollination for production and many crops have higher quality after The most effective native bees insect pollination.1,2,3 Bees are the most important pollinators in in PA and NJ and how to most ecosystems. They facilitate reproduction and improve seed set for half of Pennsylvania’s and New Jersey’s top fruit and identify them 4,5,6 vegetable commodities. Estimated value of their pollination services range from $6 - 263 million each year.7 Their habitat and foraging Honeybee numbers in Pennsylvania and New Jersey have needs been declining over the past several years. Beekeepers recorded overwinter losses of 26- 48% and 17-40% respectively in PA and Strategies for encouraging NJ between 2006 and 2009.8,9,10 These losses are much higher than their presence on your farm the typical 15% losses seen in previous years.10 Although many farmers rent managed honeybees to increase crop yield and quality, Sources of funding surveys of small to medium size PA and NJ farms have shown that native bees provide a substantial portion of pollination services.11,12 By increasing the number and diversity of native bees, PA and NJ farmers may be able to counter rising costs of rented bee colonies while supporting sustainable native plant and pollinator communities. -

Bombus Impatiens, Common Eastern Bumblebee
Bombus impatiens, Common Eastern Bumble Bee (Hymenoptera: Apidae) Christopher J. Fellows, Forest Huval, T.E. Reagan and Chris Carlton Description The common eastern bumble bee, Bombus impatiens, is an important native pollinator found in the eastern United States and southern Canada. Adult bees are covered with short, even hairs across their bodies. They are mostly black with a yellow thorax (middle section) and an additional yellow stripe at the base of the abdomen. As with other social insects, the different forms, or castes, of B. impatiens vary in size. Worker bumble bees measure from 1/3 to 2/3 of an inch in length (8.5-16 mm), while drones measure ½ to ¾ of an inch (12 to 18 mm). Queen bumble bees are the largest members of the three castes, measuring between 1/3 of an inch to 1 inch (17 and 23 mm) in length. Larvae are rarely seen and are enclosed with the nest brood cells for the duration of their A common eastern bumble bee resting on a flower. Note development. They are pale, legless grubs around 1 inch the yellow patch of hairs on the forward portion of the thorax (David Cappaert, Bugwood.org). (25 mm) in length. At least six additional species of bumble bees have been construction. Within the hollow fiber ball, the queen documented in Louisiana, with another two species possibly deposits a lump of nectar-moistened pollen. The queens present but not confirmed. Identifications are mainly based also construct honey pots near the nest entrance using on the patterns of coloration of the body hairs, but these the secretions of her wax glands. -

Creating Economically and Ecologically Sustainable Pollinator Habitat District 2 Demonstration Research Project Summary Updated for Site Visit in April 2019
Creating Economically and Ecologically Sustainable Pollinator Habitat District 2 Demonstration Research Project Summary Updated for Site Visit in April 2019 The PIs are most appreciative for identification assistance provided by: Arian Farid and Alan R. Franck, Director and former Director, resp., University of South Florida Herbarium, Tampa, FL; Edwin Bridges, Botanical and Ecological Consultant; Floyd Griffith, Botanist; and Eugene Wofford, Director, University of Tennessee Herbarium, Knoxville, TN Investigators Rick Johnstone and Robin Haggie (IVM Partners, 501-C-3 non-profit; http://www.ivmpartners.org/); Larry Porter and John Nettles (ret.), District 2 Wildflower Coordinator; Jeff Norcini, FDOT State Wildflower Specialist Cooperator Rick Owen (Imperiled Butterflies of Florida Work Group – North) Objective Evaluate a cost-effective strategy for creating habitat for pollinators/beneficial insects in the ROW beyond the back-slope. Rationale • Will aid FDOT in developing a strategy to create pollinator habitat per the federal BEE Act and FDOT’s Wildflower Program • Will demonstrate that FDOT can simultaneously • Create sustainable pollinator habitat in an economical and ecological manner • Reduce mowing costs • Part of national effort coordinated by IVM Partners, who has • Established or will establish similar projects on roadside or utility ROWS in Alabama, Arkansas, Maryland, New Mexico, Oklahoma, Idaho, Montana, Virginia, West Virginia, and Tennessee; studies previously conducted in Arizona, Delaware, Michigan, and New Jersey • Developed partnerships with US Fish & Wildlife Service, Army Corps of Engineers, US Geological Survey, New Jersey Institute of Technology, Rutgers University, Chesapeake Bay Foundation, Chesapeake Wildlife Heritage, The Navajo Nation, The Wildlife Habitat Council, The Pollinator Partnership, Progressive Solutions, Bayer Crop Sciences, Universities of Maryland, Ohio, West Virginia, and the EPA.