Mccampbell Analytical, Inc
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -

Synthesis of Bromochloromethane Using Phase Transfer Catalysis
1 SYNTHESIS OF BROMOCHLOROMETHANE USING PHASE TRANSFER CATALYSIS By LANCELOT LUCRETIUS BROOKS Baccalaureus Scientiae Honores-Chemistry, Nelson Mandela Metropolitan University A dissertation submitted in fulfillment of the requirements for the Masters Degree in Chemistry In the Faculty of Science at the NELSON MANDELA METROPOLITAN UNIVERSITY Nov. 2011 Promoter : Dr G. Dugmore Co-Promoter : Prof B. Zeelie 2 DECLARATION I, Lancelot Brooks, hereby declare that the above-mentioned treatise is my own work and that it has not previously been submitted for assessment to another University, or for another qualification. ……………………………….. ……………………….. Mr. L.L. Brooks Date 3 ACKNOWLEDGEMENTS To my promoters Dr. Gary Dugmore, and Prof. Ben Zeelie for their invaluable input, help and guidance. To NRF and NMMU for financial assistance To my parents and brothers for their love and support To Peter, Batsho, Unati, and friends in NMMU chemistry research laboratory, thank you guys. To my dearest fiancée, Natasha, a very special thank you for always being there and supporting me. Love you angel. “And we know that all things work together for good to those who love God, to those who are called according to His purpose” -Romans 8:28. 4 TABLE OF CONTENTS DECLARATION……………………………………………………………………. 2 ACKNOWLEDGEMENTS……………………………………………………………. 3 TABLE OF CONTENTS………………………………………………………………. 4 LIST OF FIGURES…………………………………………………………………….. 8 LIST OF TABLES……………………………………………………………………… 9 LIST OF EQUATIONS………………………………………………………………… 11 SUMMARY……………………………………………………………………………… 12 CHAPTER 1…………………………………………………………………………….. 14 INTRODUCTION………………………………………………………………………. 14 1.1. Technology of leather production……………………………………………….. 14 1.2. Synthesis of TCMTB……………………………………………………………… 17 1.3. Bromine……………………………………………………………………………. 20 1.3.1. Overview……………………………………………………………. 20 1.3.2. Applications of bromine compounds…………..…………………. 22 1.3.2.1. Photography……………………………………………… 22 1.3.2.2. -

Supplement to the Role of Internal Standards and Their Interaction With
Supplement to: Hiatt, M., “The Role of Internal Standards and their Interaction with Soils Impact Accuracy of Volatile Organics Determinations,” Int. J. of Environ. Anal. Chem., 2010 90:8 591-604. Please note that this is a non-copyrighted web version of supplemental information belonging to the above-cited journal article. There are formatting and page-numbering differences between this web version and the actual published version. There are also minor content differences. This version can be accessed at: http://www.epa.gov/nerlesd1/chemistry/vacuum/reference/pubs.htm This publication is also available from Informaworld: http://www.informaworld.com/smpp/content~db=all~content=a921288588 Web version, 27 April 2010 1 Supplement to: The Role of Internal Standards and their Interaction with Soils Impact Accuracy of Volatile Organics Determinations Michael H. Hiatt U.S. Environmental Protection Agency, National Exposure Research Laboratory Environmental Sciences Division. P.O. Box 93478, Las Vegas, Nevada 89193-3478 Phone: 702 798 2381. Fax: 702 798 2142. E-mail: [email protected]. Supplementary Information Data that was too detailed for “The Role of Internal Standards and their Interaction with Soils Impact Accuracy of Volatile Organics Determinations” is presented as supplemental information. This information includes each analyte by matrix and includes the accuracy evaluation, analyte results impacted by criteria, and results from varying spike equilibration times. The list of internal standards and their chemical properties are presented in Table S1 while the surrogates are listed in Table S2. Tables of results by analyte are included as Tables S3-8. These tables include the data by matrix and combined. -

An Automated Purge and Trap Gas Chromatography- Mass Spectrometry System for the Sensitive Shipboard Analysis of Volatile Organi
J. Sep. Sci. 2001, 24, 97–103 Hashimoto, Tanaka, Yamashita, Maeda97 Shinya Hashimotoa), An automated purge and trap gas chromatography- Toshiyuki Tanakab), mass spectrometry system for the sensitive Nobuyoshi Yamashitab), Tsuneaki Maedac) shipboard analysis of volatile organic compounds in seawater a) Department of Ocean Sciences, Tokyo University of We developed an automated purge and trap unit connected to a gas chromatograph- Fisheries, 4-5-7 Konan, Minato- mass spectrometer for shipboard determination of unstable volatile organic com- ku, Tokyo 108±8477, Japan pounds in seawater. The device used a small column for the rapid desorption of ad- b) National Institute for sorbed compounds, thus eliminating the need for post-desorption cryofocusing. The Resources and Environment, a 16-3 Onogawa, Tsukuba, repeatability (relative standard deviation, RSD; n = 7) was typically 5%. The detection Ibaraki 305±8569, Japan limits were 0.1–4.3 pM for chloromethane, bromomethane, dichloromethane, iodo- c) DKK Corporation, 4-13-14 methane, dimethyl sulfide, iodoethane, isoprene, bromochloromethane, chloroform, Kichijoji Kitamachi, Musashino- tetrachloromethane, dibromomethane, bromodichloromethane, iodopropane, chlor- shi, Tokyo 108-0001, Japan oiodomethane, dimethyl disulfide, dibromochloromethane, bromoform, and diiodo- methane. To investigate the stability of seawater samples, we obtained a concentra- tion-time profile of volatile organic compounds using this method during the incubation of a seawater sample with and without the addition of HgCl2 in the dark at 48C. We found shipboard determination to be suitable and essential for the determination of un- stable compounds such as dimethyl sulfide in seawater, as the concentration of di- methyl sulfide increased considerably during the incubation of a seawater sample both with and without the addition of HgCl2. -

SROC Annex V
Annex V Major Chemical Formulae and Nomenclature This annex presents the formulae and nomenclature for halogen-containing species and other species that are referred to in this report (Annex V.1). The nomenclature for refrigerants and refrigerant blends is given in Annex V.2. V.1 Substances by Groupings V.1.1 Halogen-Containing Species V.1.1.1 Inorganic Halogen-Containing Species Atomic chlorine Cl Atomic bromine Br Molecular chlorine Cl2 Molecular bromine Br2 Chlorine monoxide ClO Bromine monoxide BrO Chlorine radicals ClOx Bromine radicals BrOx Chloroperoxy radical ClOO Bromine nitrate BrONO2, BrNO3 Dichlorine peroxide (ClO dimer) (ClO)2, Cl2O2 Potassium bromide KBr Hydrogen chloride (Hydrochloric acid) HCl Inorganic chlorine Cly Antimony pentachloride SbCl5 Atomic fluorine F Molecular fluorine F2 Atomic iodine I Hydrogen fluoride (Hydrofluoric acid) HF Molecular iodine I2 Sulphur hexafluoride SF6 Nitrogen trifluoride NF3 IPCC Boek (dik).indb 467 15-08-2005 10:57:13 468 IPCC/TEAP Special Report: Safeguarding the Ozone Layer and the Global Climate System V.1.1.2 Halocarbons For each halocarbon the following information is given in columns: • Chemical compound [Number of isomers]1 (or common name) • Chemical formula • CAS number2 • Chemical name (or alternative name) V.1.1.2.1 Chlorofluorocarbons (CFCs) CFC-11 CCl3F 75-69-4 Trichlorofluoromethane CFC-12 CCl2F2 75-71-8 Dichlorodifluoromethane CFC-13 CClF3 75-72-9 Chlorotrifluoromethane CFC-113 [2] C2Cl3F3 Trichlorotrifluoroethane CCl FCClF 76-13-1 CFC-113 2 2 1,1,2-Trichloro-1,2,2-trifluoroethane -

ACE Tips for Filing EPA ODS
Automated Commercial Environment ACE Tips for Filing EPA ODS June, 2018 Ozone-depleting substances (ODS) deplete the stratospheric ozone layer when the chlorine and bromine atoms that they contain come into contact with ozone molecules. One chlorine atom can destroy over 100,000 ozone molecules before it is removed from the stratosphere. ODS that release chlorine include chlorofluorocarbons (CFCs), hydrochlorofluorocarbons (HCFCs), carbon tetrachloride, and methyl chloroform. ODS that release bromine include halons and methyl bromide. HTS code Ozone-Depleting Substance 2903.14.0000 Carbon tetrachloride What commodities 2903.19.6010 Methylchloroform (1,1,1-Trichloroethane) are ODS used in? 2903.39.1520 Methyl bromide 2903.71.0000 Chlorodifluoromethane (HCFC-22) ODS have been used 2903.72.0020 Dichlorotrifluoroethane (HCFC-123) for refrigeration, air 2903.73.0000 Dichlorofluoroethanes (HCFC-141, 141b) conditioning, insulation, 2903.74.0000 Chlorodifluoroethanes (HCFC-142, 142b) solvents, aerosol 2903.75.0000 Dichloropentafluoropropanes propellants, and in other (HCFC-225, 225ca, 225cb) sectors. Currently, the United 2903.76.0010 Bromotrifluoromethane (Halon 1301) States is in the 2903.76.0050 Bromochlorodifluoromethane (Halon 1211), process of phasing bromotrifluoromethane (Halon 1301), and out HCFCs, but in the dibromotetrafluoroethanes (Halon 2402), Other interim limited imports are 2903.77.0010 Trichlorofluoromethane (CFC-11) allowed. CFCs, 2903.77.0020 Trichlorotrifluoroethanes (CFC-113, CFC-113a) methyl bromide, halon, 2903.77.0030 -

Maine Remedial Action Guidelines (Rags) for Contaminated Sites
Maine Department of Environmental Protection Remedial Action Guidelines for Contaminated Sites (RAGs) Effective Date: May 1, 2021 Approved by: ___________________________ Date: April 27, 2021 David Burns, Director Bureau of Remediation & Waste Management Executive Summary MAINE DEPARTMENT OF ENVIRONMENTAL PROTECTION 17 State House Station | Augusta, Maine 04333-0017 www.maine.gov/dep Maine Department of Environmental Protection Remedial Action Guidelines for Contaminated Sites Contents 1 Disclaimer ...................................................................................................................... 1 2 Introduction and Purpose ............................................................................................... 1 2.1 Purpose ......................................................................................................................................... 1 2.2 Consistency with Superfund Risk Assessment .............................................................................. 1 2.3 When to Use RAGs and When to Develop a Site-Specific Risk Assessment ................................. 1 3 Applicability ................................................................................................................... 2 3.1 Applicable Programs & DEP Approval Process ............................................................................. 2 3.1.1 Uncontrolled Hazardous Substance Sites ............................................................................. 2 3.1.2 Voluntary Response Action Program -

Hal-List.Pdf
Florida Department of Health Environmental Health Bureau of Environmental Health, Water Programs Maximum Contaminant Levels and Health Advisory Levels Last Update 8/4/2016 HAL = Health Advisory Level MCL = Maximum Contaminant Level SMCL = Secondary Maximum Contaminant Level ug/L = micrograms per Liter or parts per billion mg/L = milligrams per Liter or parts per million MFL = million fibers per liter Chemical Name Units MCL/HAL 1,1,1,2‐TETRACHLOROETHANE 1 ug/L HAL ALPHA‐BHC 0.04 ug/L HAL CHLORPROPHAM 1400 ug/L HAL GLYPHOSATE 700 ug/L MCL PENTACHLOROPHENOL 1 ug/L MCL 1,1,1‐TRICHLOROETHANE 200 ug/L MCL 1,1,2,2‐TETRACHLOROETHANE 1 ug/L HAL 1,1,2‐TRICHLOROETHANE 5 ug/L MCL 1,1‐DICHLOROETHANE 70 ug/L HAL 1,1‐DICHLOROETHYLENE 7 ug/L MCL 1,2,3‐TRICHLOROBENZENE 70 ug/L HAL 1,2,3‐TRICHLOROPROPANE 40 ug/L HAL 1,2,4‐TRICHLOROBENZENE 70 ug/L MCL 1,2,4‐TRIMETHYLBENZENE 35 ug/L HAL 1,2‐DICHLOROETHANE 3 ug/L MCL 1,2‐DICHLOROPROPANE 5 ug/L MCL 1,3,5‐TRIMETHYLBENZENE 35 ug/L HAL 1,4‐DIOXANE 0.35 ug/L HAL 1‐CHLOROBUTANE 2800 ug/L HAL 2,3,7,8 TCDD (DIOXIN) 0.00003 ug/L MCL 2,4,5‐T 70 ug/L HAL 2,4,5‐TP (SILVEX) 50 ug/L MCL 2,4‐D 70 ug/L MCL 2,4‐DB 56 ug/L HAL 2,4‐DINITROTOLUENE 0.05 ug/L HAL 2,6‐DINITROTOLUENE 0.05 ug/L HAL 2‐BUTANONE 4200 ug/L HAL 2‐HEXANONE 280 ug/L HAL 4,4'‐DDD 0.1 ug/L HAL 4,4'‐DDE 0.1 ug/L HAL 4,4'‐DDT 0.1 ug/L HAL 4‐NITROPHENOL 56 ug/L HAL 8/4/2016 Chemical Name Units MCL/HAL ACENAPHTHYLENE 210 ug/L HAL ACIFLUORFEN 1 ug/L HAL ALACHLOR 2 ug/L MCL ALDICARB (SUM OF ALL ALDICARBS) 7 ug/L HAL ALDICARB SULFONE (SUM OF ALDICARBS) 7 ug/L HAL -

The Alkylation of the Boron Hydrides, Reaction of Pentaborane-9 with 1
This dissertation has been 62-829 microfilmed exactly as received ! ALTWICKER, Elmar Robert, 1930- j THE ALKYLATION OF THE BORON HYDRIDES; REACTION OF PENTABOlLANE-9 WITH 1-BUTENE AND SOME POLYHALOGENATED HYDROCARBONS. The Ohio State University, Ph.D., 1957 Chemistry, organic University Microfilms, Inc., Ann Arbor, Michigan THE ALKYLATION OF THE BORON HYDRIDES Reaction of Pentaborane-9 with 1-Butene and Some Polyhalogenated Hydrocarbons DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University EIZvIAR RP'^TWIGKER, B.S. The Ohio State University 1957 Approved by; m Departme Ï Chemistry Acknowledgement The author would like to thank Professor A.B, Garrett for the supervision of this research. He would also lik e to thank Dr. John Norman of the Olin-Mathieson Chemical Corporation for the mass spectrographic analyses. 11 Table of Contents Page I. Purpose .................................................... ...... 1 II. Historical ..................... ........... 2 III, Experimental ....................................... 5 A. Apparatus ............................ ......... $ 1. Main Vacuum System .................... ... ^ 2. Reaction-, Transfer-, and Storage A pparatus............................................................ ^ 3. Analytical Apparatus .......... Vy 5. Materials l 6 G. Reaction of Pentaborane-9 with 1-Butene . 18 1. Reaction and Separation of the Reaction Mixture ............ 19 2. Identification of Butylpentaborane . 2h 3. -

Global Warming Potentials (Gwps), and Global Temperature Change Potentials (Gtps)
APPENDIX A SUMMARY OF ABUNDANCES, LIFETIMES, OZONE DEPLETION POTENTIALS (ODPS), RADIATIVE EFFICIENCIES (RES), GLOBAL WARMING POTENTIALS (GWPS), AND GLOBAL TEMPERATURE CHANGE POTENTIALS (GTPS) Lead Author J.B. Burkholder Contributors Ø. Hodnebrog V.L. Orkin 2 Cover photo: Experimental apparatus used in fundamental kinetic and photochemical laboratory studies. Lab- oratory measurements provide key input to the derivation of the parameters reported in this appendix. Photo: W. von Dauster, NOAA. APPENDIX A SUMMARY OF ABUNDANCES, LIFETIMES, OZONE DEPLETION POTENTIALS (ODPS), RADIATIVE EFFICIENCIES (RES), GLOBAL WARMING POTENTIALS (GWPS), AND GLOBAL TEMPERATURE CHANGE POTENTIALS (GTPS) CONTENTS APPENDIX A: INTRODUCTION . 1 APPENDIX A: SUMMARY OF ABUNDANCES, LIFETIMES, ODPS, RES, GWPS, AND GTPS . 2 Hydrocarbons . 2 Oxygenated Hydrocarbons . 2 Chlorofluorocarbons . 2 Hydrochlorofluorocarbons . 4 Hydrofluorocarbons . 18 Unsaturated Hydrofluorocarbons . 20 Chlorocarbons and Hydrochlorocarbons . 22 Unsaturated Hydrochlorocarbons and Chlorocarbons . 24 Unsaturated Chlorofluorocarbons and Hydrochlorofluorocarbons . 24 Bromocarbons, Hydrobromocarbons and Halons . 24 Unsaturated Bromofluorocarbons . 26 Unsaturated Bromochlorofluorocarbons . 26 Fully Fluorinated Species . 26 Halogenated Ethers . 28 Fluoroesters . 32 Halogenated Alcohols . 34 Halogenated Ketones . 36 Iodocarbons . 36 Special Compounds . 36 Table Heading Footnotes . 38 Abundance Footnotes . 39 Lifetime Footnotes . 39 ODP Footnotes . 42 RE, GWP, and GTP Footnotes . 43 REFERENCES . 44 This -

Burske Norbert W 195708 Phd
"In presenting the dissertation as a partial fulfillment of the requirements for an advanced degree from the Georgia Institute of Technology, I agree that the Library of the Institution shall make it available for inspection and circulation in accordance with its regulations governing materials of this type. I agree that permission to copy from, or to publish from, this dissertation may be granted by the professor under whose direction it was written, or, in his absence, by the dean of the Graduate Division when such copying or publication is solely for scholarly purposes and does not involve potential financial gain. It is understood that any copying from, or publication of, this dissertation which involves potential financial gain will not be allowed without written permission. n It THE KINETICS OF THE BASE-CATALYZED DEUTERIUM EXCHANGE OF SOME HALOFORMS IN AQUEOUS SOLUTION A THESIS Presented to the Faculty of the Graduate Division Georgia Institute of Technology In Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the School of Chemistry By Norbert William Burske June 1957 1< THE KINETICS OF THE BASE-CATALYZED DEUTERIUM EXCHANGE OF SOME HALOFORMS IN AQUEOUS SOLUTION Approved, J I ki Jack Hine I ,r Lod D. Frashier Erlft.Edg Grovenstein, Jr. Date Approved by Chairman, ii ACKNOWLEDGEMENT The author wishes to gratefully acknowledge his in- debtedness to Dr. J. Hine for invaluable guidance and without his aid this project would never have been completed. Also, the author is indebted to the Atomic Energy Commission and the Office of Ordnance Research, U. S. Army for sponsoring assistantships. -
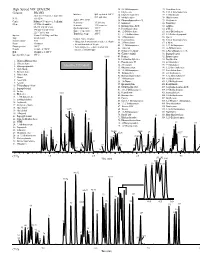
GC and GC/MS Applications Environmental-Volatiles
GC and GC/MS Applications Environmental-Volatiles High Speed VOC EPA 8260 32. 2,2-Dichloropropane 75. Tetrachloroethene Column: DB-VRX 33. Ethyl acetate 76. 1,1,1,2-Tetrachloroethane Interface: Split injector at 150°C, 34. Ethyl-tert-butyl ether 77. 1-Chlorohexane 20 m x 0.18 mm id, 1.0 µm film 60:1 split ratio P/N: 121-1524 35. Methyl acrylate 78. Chlorobenzene Agilent 5973 MSD 36. Dibromofluoromethane IS 79. Ethylbenzene Carrier: Helium at 55 cm/sec (1.5 mL/min) Scan range: 35-260 amu Oven: 45°C for 3.0 minutes 37. Isobutanol 80. Bromoform Scan rate: 3.25 scans/sec 38. Dichloroethane-d4 IS 81. m-Xylene 45-190°C at 36°C/min Quad temperature: 150°C 190-225°C at 20°C/min 39. Pentafluorobenzene 82. p-Xylene Source temperature: 200°C 40. 1,2-Dichloroethane 83. trans-Dichlorobutene 225°C for 0.5 min Transfer line temp: 200°C Injector: Tekmar 3100 Purge and Trap 41. 1,1,1-Trichloroethane 84. 1,3-Dichloro-2-propanol 42. 1-Chlorobutane 85. Styrene Trap: Vocarb 3000 Sample Concentration Sample volume: 5 mL 43. Crotonaldehyde 86. 1,1,2,2-Tetrachloroethane • Halogentated and aromatic analytes at 40 ppb 44. 2-Chloroethanol 87. o-Xylene Purge: 11 minutes • Internal standards at 20 ppb Desorb preheat: 245°C 45. 1,1-Dichloropropene 88. 1,2,3-Trichlropropane • Polar analytes (i.e., ethers, alcohols and 46. 1-Butanol 89. cis-Dichlorobutene Desorb: 1 minute at 250°C ketones at 100-800 ppb) Bake: 10 minutes at 260°C 47.