GC and GC/MS Applications Environmental-Volatiles
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Interstellar Ices and Radiation-Induced Oxidations of Alcohols
The Astrophysical Journal, 857:89 (8pp), 2018 April 20 https://doi.org/10.3847/1538-4357/aab708 © 2018. The American Astronomical Society. All rights reserved. Interstellar Ices and Radiation-induced Oxidations of Alcohols R. L. Hudson and M. H. Moore Astrochemistry Laboratory, NASA Goddard Space Flight Center, Greenbelt, Maryland, 20771, USA; [email protected] Received 2017 May 2; revised 2018 March 8; accepted 2018 March 9; published 2018 April 18 Abstract Infrared spectra of ices containing alcohols that are known or potential interstellar molecules are examined before and after irradiation with 1 MeV protons at ∼20 K. The low-temperature oxidation (hydrogen loss) of six alcohols is followed, and conclusions are drawn based on the results. The formation of reaction products is discussed in terms of the literature on the radiation chemistry of alcohols and a systematic variation in their structures. The results from these new laboratory measurements are then applied to a recent study of propargyl alcohol. Connections are drawn between known interstellar molecules, and several new reaction products in interstellar ices are predicted. Key words: astrochemistry – infrared: ISM – ISM: molecules – molecular data – molecular processes 1. Introduction 668 cm−1 was produced in solid propargyl alcohol’sIR spectrum after the compound was irradiated with 2 keV Interstellar ices are now recognized as an important comp- electrons (Sivaraman et al. 2015, hereafter SMSB from the onent of the interstellar medium (ISM). The results of multiple coauthors final initials). That IR peak was assigned to benzene infrared (IR) surveys have led to over a dozen assignments of (C H ), which was said “to be the major product from spectral features to molecular ices, with the more abundant 6 6 propargyl alcohol irradiation.” However, a full mid-IR interstellar ices being H O, CO, and CO (Boogert et al. -

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -

Synthesis of Bromochloromethane Using Phase Transfer Catalysis
1 SYNTHESIS OF BROMOCHLOROMETHANE USING PHASE TRANSFER CATALYSIS By LANCELOT LUCRETIUS BROOKS Baccalaureus Scientiae Honores-Chemistry, Nelson Mandela Metropolitan University A dissertation submitted in fulfillment of the requirements for the Masters Degree in Chemistry In the Faculty of Science at the NELSON MANDELA METROPOLITAN UNIVERSITY Nov. 2011 Promoter : Dr G. Dugmore Co-Promoter : Prof B. Zeelie 2 DECLARATION I, Lancelot Brooks, hereby declare that the above-mentioned treatise is my own work and that it has not previously been submitted for assessment to another University, or for another qualification. ……………………………….. ……………………….. Mr. L.L. Brooks Date 3 ACKNOWLEDGEMENTS To my promoters Dr. Gary Dugmore, and Prof. Ben Zeelie for their invaluable input, help and guidance. To NRF and NMMU for financial assistance To my parents and brothers for their love and support To Peter, Batsho, Unati, and friends in NMMU chemistry research laboratory, thank you guys. To my dearest fiancée, Natasha, a very special thank you for always being there and supporting me. Love you angel. “And we know that all things work together for good to those who love God, to those who are called according to His purpose” -Romans 8:28. 4 TABLE OF CONTENTS DECLARATION……………………………………………………………………. 2 ACKNOWLEDGEMENTS……………………………………………………………. 3 TABLE OF CONTENTS………………………………………………………………. 4 LIST OF FIGURES…………………………………………………………………….. 8 LIST OF TABLES……………………………………………………………………… 9 LIST OF EQUATIONS………………………………………………………………… 11 SUMMARY……………………………………………………………………………… 12 CHAPTER 1…………………………………………………………………………….. 14 INTRODUCTION………………………………………………………………………. 14 1.1. Technology of leather production……………………………………………….. 14 1.2. Synthesis of TCMTB……………………………………………………………… 17 1.3. Bromine……………………………………………………………………………. 20 1.3.1. Overview……………………………………………………………. 20 1.3.2. Applications of bromine compounds…………..…………………. 22 1.3.2.1. Photography……………………………………………… 22 1.3.2.2. -

Supplement to the Role of Internal Standards and Their Interaction With
Supplement to: Hiatt, M., “The Role of Internal Standards and their Interaction with Soils Impact Accuracy of Volatile Organics Determinations,” Int. J. of Environ. Anal. Chem., 2010 90:8 591-604. Please note that this is a non-copyrighted web version of supplemental information belonging to the above-cited journal article. There are formatting and page-numbering differences between this web version and the actual published version. There are also minor content differences. This version can be accessed at: http://www.epa.gov/nerlesd1/chemistry/vacuum/reference/pubs.htm This publication is also available from Informaworld: http://www.informaworld.com/smpp/content~db=all~content=a921288588 Web version, 27 April 2010 1 Supplement to: The Role of Internal Standards and their Interaction with Soils Impact Accuracy of Volatile Organics Determinations Michael H. Hiatt U.S. Environmental Protection Agency, National Exposure Research Laboratory Environmental Sciences Division. P.O. Box 93478, Las Vegas, Nevada 89193-3478 Phone: 702 798 2381. Fax: 702 798 2142. E-mail: [email protected]. Supplementary Information Data that was too detailed for “The Role of Internal Standards and their Interaction with Soils Impact Accuracy of Volatile Organics Determinations” is presented as supplemental information. This information includes each analyte by matrix and includes the accuracy evaluation, analyte results impacted by criteria, and results from varying spike equilibration times. The list of internal standards and their chemical properties are presented in Table S1 while the surrogates are listed in Table S2. Tables of results by analyte are included as Tables S3-8. These tables include the data by matrix and combined. -

Adducts of Propargyl Alcohol and Their Use As Corrosion Inhibitors in Acidizing Systems
Europaisches Patentamt 19) s European Patent Office © Publication number: 0 239 770 Office europeen des brevets A1 © EUROPEAN PATENT APPLICATION © Application number: 87102356.0 © Int. CI.3: C 23 F 11/12 E 21 B 41/02, E 21 B 37/06 © Date of filing: 19.02.87 © Priority: 28.02.86 US 834526 © Applicant: BASF Corporation 9 Campus Drive Parsippany, NJ 07054(US) © Date of publication of application: 07.10.87 Bulletin 87/41 © Inventor: Perry, Christine 22335 Nixon © Designated Contracting States: Riverview Michigan 481 92(US) DE FR GB NL © Inventor: Crema,Stefano Carlo 1874 20th Street Wyandotte Michigan 48192(US) © Inventor: Davis, Pauls 30027 White Gibraltar Michigan 48173(US) @ Representative: Holler, Klaus, Dr. et al, BASF Aktiengesellschaft Carl-Bosch-Strasse 38 D-6700 Ludwigshafen(DE) © Adducts of propargyl alcohol and their use as corrosion inhibitors in acidizing systems. © The invention relates to an acidizing system comprising: (a) an acidizing solution; and (b) an effective corrosion inhibiting amount of a prop- argyl alcohol adduct having the following structural formula I: _ _ HC a C-CH2-0- -(CH2)5C-^ -H (I) wherein n = 1 or 3 and R = H if n = 3 and R = CH3 if n = 1 ; and x = 1 to 5. Corrosion of ferrous metals is inhibited by treating the surface thereof with the acidizing system. o IN M CM 0. Ul Croydon Printing Company Ltd. BASF Corporation ADDUCTS OF PROPARGYL ALCOHOL AND THEIR USE AS CORROSION INHIBITORS IN ACIDIZING SYSTEMS This invention relates to acidizing systems for oil and gas recovery containing propylene oxide and/or butylene oxide adducts of propargyl alcohol as corrosion inhibitors. -

Chemical Name Federal P Code CAS Registry Number Acutely
Acutely / Extremely Hazardous Waste List Federal P CAS Registry Acutely / Extremely Chemical Name Code Number Hazardous 4,7-Methano-1H-indene, 1,4,5,6,7,8,8-heptachloro-3a,4,7,7a-tetrahydro- P059 76-44-8 Acutely Hazardous 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- hexachloro-1,5,5a,6,9,9a-hexahydro-, 3-oxide P050 115-29-7 Acutely Hazardous Methanimidamide, N,N-dimethyl-N'-[2-methyl-4-[[(methylamino)carbonyl]oxy]phenyl]- P197 17702-57-7 Acutely Hazardous 1-(o-Chlorophenyl)thiourea P026 5344-82-1 Acutely Hazardous 1-(o-Chlorophenyl)thiourea 5344-82-1 Extremely Hazardous 1,1,1-Trichloro-2, -bis(p-methoxyphenyl)ethane Extremely Hazardous 1,1a,2,2,3,3a,4,5,5,5a,5b,6-Dodecachlorooctahydro-1,3,4-metheno-1H-cyclobuta (cd) pentalene, Dechlorane Extremely Hazardous 1,1a,3,3a,4,5,5,5a,5b,6-Decachloro--octahydro-1,2,4-metheno-2H-cyclobuta (cd) pentalen-2- one, chlorecone Extremely Hazardous 1,1-Dimethylhydrazine 57-14-7 Extremely Hazardous 1,2,3,4,10,10-Hexachloro-6,7-epoxy-1,4,4,4a,5,6,7,8,8a-octahydro-1,4-endo-endo-5,8- dimethanonaph-thalene Extremely Hazardous 1,2,3-Propanetriol, trinitrate P081 55-63-0 Acutely Hazardous 1,2,3-Propanetriol, trinitrate 55-63-0 Extremely Hazardous 1,2,4,5,6,7,8,8-Octachloro-4,7-methano-3a,4,7,7a-tetra- hydro- indane Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]- 51-43-4 Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, P042 51-43-4 Acutely Hazardous 1,2-Dibromo-3-chloropropane 96-12-8 Extremely Hazardous 1,2-Propylenimine P067 75-55-8 Acutely Hazardous 1,2-Propylenimine 75-55-8 Extremely Hazardous 1,3,4,5,6,7,8,8-Octachloro-1,3,3a,4,7,7a-hexahydro-4,7-methanoisobenzofuran Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime 26419-73-8 Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime. -
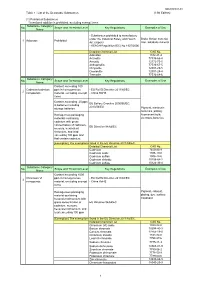
Table 1 : List of the Declarable Substances (1) Prohibited
DQ2000001-03 Table 1 : List of the Declarable Substances (13th Edition) (1) Prohibited Substances *Intentional addition is prohibited, excluding exempt items Substance Category/ No. Scope and Threshold Level Key Regulations Examples of Use Name - Substances prohibited to manufacture under the Industrial Safety and Health Brake friction material, 1 Asbestos Prohibited Act (Japan) filler, adiabatic material - REACH Regulation (EC) No 1907/2006 Detailed Chemical List CAS No. Asbestos 1332-21-4 Actinolite 77536-66-4 Amosite 12172-73-5 Anthophyllite 77536-67-5 Chrysotile 12001-29-5 Crocidolite 12001-28-4 Tremolite 77536-68-6 Substance Category/ No. Scope and Threshold Level Key Regulations Examples of Use Name Content exceeding 100 Cadmium/cadmium ppm in homogeneous - EU RoHS Directive 2011/65/EC 2 compounds material, excluding exempt - China RoHS items Content exceeding 20 ppm EU Battery Directive 2006/66/EC, in batteries including 2013/56/EU storage batteries Pigment, electronic materials, plating, Homogenous packaging fluorescent bulb, materials containing electrode,batteries cadmium with gross concentration of cadmium, EU Directive 94/62/EC mercury, hexavalent chromium, and lead exceeding 100 ppm and that contain cadmium [Exemption] The exemptions listed in the EU Directive 2011/65/EC. Detailed Chemical List CAS No. Cadmium 7440-43-9 Cadmium oxide 1306-19-0 Cadmium sulfide 1306-23-6 Cadmium chloride 10108-64-2 Cadmium sulfate 10124-36-4 Substance Category/ No. Scope and Threshold Level Key Regulations Examples of Use Name Content exceeding 1000 Chromium VI ppm in homogeneous - EU RoHS Directive 2011/65/EC 3 compounds material, excluding exempt - China RoHS items Homogenous packaging Pigment, catalyst, material containing plating, dye, surface hexavalent chromium with treatment gross concentration of EU Directive 94/62/EC cadmium, mercury, hexavalent chromium, and lead exceeding 100 ppm [Exemption] The exemptions listed in the EU Directive 2011/65/EC. -

Synthesis of Propargyl Alcohol Author(S)
Studies on ethinylation reactions, II : synthesis of propargyl Title alcohol Author(s) Suzuki, Keizo The Review of Physical Chemistry of Japan (1954), 23(2): 66- Citation 72 Issue Date 1954-02-15 URL http://hdl.handle.net/2433/46699 Right Type Departmental Bulletin Paper Textversion publisher Kyoto University The Review of Physical Chemistry of Japan Vol. 23 No. 2 (1953) STUDIES ON ETHINYLATION REACTIONS, II Synthesis of Propargyl Alcohol By Ka¢c~ Sczcxi Introduction Propargyl alcohol is usually produced as the intermediate when butynediol-1, 4 is synthesized from acetylene and fornaldehyde, but it is to be possible to obtain propargyl alcohol as the main product if the conversion to butynediol can be checked by control- ling suitably the reaction conditions. W. Reppeil has obtained the satisfactory results in the synthesis of propargyl alcohol in a continuous process, while the unsuccessful result has been reported so far as a continuous process is adoptedzl. Therefore, it is expected that the synthesis of propargyl alcohol as the main product is difficult in com- parison with the case of butynediol. From the previous studies on the kinetics of the reaction of acetylene with aqueous formaldehyde solutiona~^>and the synthesis of butynediol in a continuous process sl, the results of the investigations on the propargyl alcohol synthesis are summarized as follows. The rate, the apparent equilibrium concentration of propargyl alcohol and the ratio in moles of the quantity of the propargyl alcohol formed to that of formaldehyde consumed, PfF increase when acetylene pressure is raised. In the cases where pH is low and the methanol content contained in an aqueous formaldehyde solution is high, the concentration of propargyl alcohol formed and P/F come to exceed the cases where pH is high and the methanol content is low, though the reaction rate is slow at the earlier stage. -

Acutely / Extremely Hazardous Waste List
Acutely / Extremely Hazardous Waste List Federal P CAS Registry Acutely / Extremely Chemical Name Code Number Hazardous 4,7-Methano-1H-indene, 1,4,5,6,7,8,8-heptachloro-3a,4,7,7a-tetrahydro- P059 76-44-8 Acutely Hazardous 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- hexachloro-1,5,5a,6,9,9a-hexahydro-, 3-oxide P050 115-29-7 Acutely Hazardous Methanimidamide, N,N-dimethyl-N'-[2-methyl-4-[[(methylamino)carbonyl]oxy]phenyl]- P197 17702-57-7 Acutely Hazardous 1-(o-Chlorophenyl)thiourea P026 5344-82-1 Acutely Hazardous 1-(o-Chlorophenyl)thiourea 5344-82-1 Extemely Hazardous 1,1,1-Trichloro-2, -bis(p-methoxyphenyl)ethane Extemely Hazardous 1,1a,2,2,3,3a,4,5,5,5a,5b,6-Dodecachlorooctahydro-1,3,4-metheno-1H-cyclobuta (cd) pentalene, Dechlorane Extemely Hazardous 1,1a,3,3a,4,5,5,5a,5b,6-Decachloro--octahydro-1,2,4-metheno-2H-cyclobuta (cd) pentalen-2- one, chlorecone Extemely Hazardous 1,1-Dimethylhydrazine 57-14-7 Extemely Hazardous 1,2,3,4,10,10-Hexachloro-6,7-epoxy-1,4,4,4a,5,6,7,8,8a-octahydro-1,4-endo-endo-5,8- dimethanonaph-thalene Extemely Hazardous 1,2,3-Propanetriol, trinitrate P081 55-63-0 Acutely Hazardous 1,2,3-Propanetriol, trinitrate 55-63-0 Extemely Hazardous 1,2,4,5,6,7,8,8-Octachloro-4,7-methano-3a,4,7,7a-tetra- hydro- indane Extemely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]- 51-43-4 Extemely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, P042 51-43-4 Acutely Hazardous 1,2-Dibromo-3-chloropropane 96-12-8 Extemely Hazardous 1,2-Propylenimine P067 75-55-8 Acutely Hazardous 1,2-Propylenimine 75-55-8 Extemely Hazardous 1,3,4,5,6,7,8,8-Octachloro-1,3,3a,4,7,7a-hexahydro-4,7-methanoisobenzofuran Extemely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime 26419-73-8 Extemely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime. -

Predicting OH Stretching Fundamental Wavenumbers of Alcohols for Conformational Cite This: Phys
PCCP View Article Online PAPER View Journal | View Issue Predicting OH stretching fundamental wavenumbers of alcohols for conformational Cite this: Phys. Chem. Chem. Phys., 2021, 23, 5629 assignment: different correction patterns for density functional and wave-function-based methods† Robert Medel * and Martin A. Suhm A model is presented for the prediction of OH stretching fundamental wavenumbers of alcohol conformers in the gas phase by application of a small set of empirical anharmonicity corrections to calculations in the harmonic approximation. In contrast to the popular application of a uniform scaling factor, the local chemical structure of the alcohol is taken into account to greatly improve accuracy. Interestingly, different correction patterns emerge for results of hybrid density functional (B3LYP-D3 and PBE0-D3) and wave-function-based Creative Commons Attribution 3.0 Unported Licence. methods (SCS-LMP2, LCCSD(T*)-F12a and CCSD(T)-F12a 1D). This raises questions about electronic structure deficiencies in these methods and differences in anharmonicity between alcohols. After its initial construction on the basis of literature assignments the model is tested with Raman jet spectroscopy of propargyl alcohol, Received 24th January 2021, cyclohexanol, borneol, isopinocampheol and 2-methylbutan-2-ol. For propargyl alcohol a spectral splitting Accepted 24th February 2021 attributed to tunneling is resolved. PBE0-D3 is identified as a well performing and broadly affordable À1 DOI: 10.1039/d1cp00342a electronic structure method for this model. A mean absolute error of 1.3 cm and a maximum absolute error of 3 cmÀ1 result for 46 conformers of 24 alcohols in a 60 cmÀ1 range, when a single parameter is rsc.li/pccp adjusted separately for each alcohol substitution class(methanol,primary,secondary,tertiary). -

Synthesis and Cytotoxicity Evaluation of Small
Synthesis and Cytotoxicity evaluation of small 1,4 - triazolic derivatives against B16 melanoma cell lines and a methodolgy study on the synthesis of propargyl ethers from their corresponding propargyl esters without catalyst and under microwave irradiations Shiva Kalhor Monfared To cite this version: Shiva Kalhor Monfared. Synthesis and Cytotoxicity evaluation of small 1,4 - triazolic derivatives against B16 melanoma cell lines and a methodolgy study on the synthesis of propargyl ethers from their corresponding propargyl esters without catalyst and under microwave irradiations. Organic chemistry. Université Pierre et Marie Curie - Paris VI, 2014. English. NNT : 2014PA066235. tel- 01133657 HAL Id: tel-01133657 https://tel.archives-ouvertes.fr/tel-01133657 Submitted on 20 Mar 2015 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. THESE DE DOCTORAT DE L’UNIVERSITE PIERRE ET MARIE CURIE Spécialité Chimie Organique Ecole doctorale de Chimie Moléculaire Paris Centre Présentée par Mme Shiva Kalhor-Monfared Pour obtenir le grade de DOCTEUR de l’UNIVERSITÉ PIERRE ET MARIE CURIE Sujet de la thèse : Synthesis and Cytotoxicity evaluation of small 1,4-triazolic derivatives against B16 melanoma cell lines and a methodolgy study on the synthesis of propargyl ethers from their corresponding propargyl esters without catalyst and under microwave irradiations soutenue le 18 septembre 2014 devant le jury composé de : Mr F. -

SROC Annex V
Annex V Major Chemical Formulae and Nomenclature This annex presents the formulae and nomenclature for halogen-containing species and other species that are referred to in this report (Annex V.1). The nomenclature for refrigerants and refrigerant blends is given in Annex V.2. V.1 Substances by Groupings V.1.1 Halogen-Containing Species V.1.1.1 Inorganic Halogen-Containing Species Atomic chlorine Cl Atomic bromine Br Molecular chlorine Cl2 Molecular bromine Br2 Chlorine monoxide ClO Bromine monoxide BrO Chlorine radicals ClOx Bromine radicals BrOx Chloroperoxy radical ClOO Bromine nitrate BrONO2, BrNO3 Dichlorine peroxide (ClO dimer) (ClO)2, Cl2O2 Potassium bromide KBr Hydrogen chloride (Hydrochloric acid) HCl Inorganic chlorine Cly Antimony pentachloride SbCl5 Atomic fluorine F Molecular fluorine F2 Atomic iodine I Hydrogen fluoride (Hydrofluoric acid) HF Molecular iodine I2 Sulphur hexafluoride SF6 Nitrogen trifluoride NF3 IPCC Boek (dik).indb 467 15-08-2005 10:57:13 468 IPCC/TEAP Special Report: Safeguarding the Ozone Layer and the Global Climate System V.1.1.2 Halocarbons For each halocarbon the following information is given in columns: • Chemical compound [Number of isomers]1 (or common name) • Chemical formula • CAS number2 • Chemical name (or alternative name) V.1.1.2.1 Chlorofluorocarbons (CFCs) CFC-11 CCl3F 75-69-4 Trichlorofluoromethane CFC-12 CCl2F2 75-71-8 Dichlorodifluoromethane CFC-13 CClF3 75-72-9 Chlorotrifluoromethane CFC-113 [2] C2Cl3F3 Trichlorotrifluoroethane CCl FCClF 76-13-1 CFC-113 2 2 1,1,2-Trichloro-1,2,2-trifluoroethane