Synthesis and Flame Retardant Studies of Bromoester of 2,4-Pentadienoic Acid
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -

Synthesis of Bromochloromethane Using Phase Transfer Catalysis
1 SYNTHESIS OF BROMOCHLOROMETHANE USING PHASE TRANSFER CATALYSIS By LANCELOT LUCRETIUS BROOKS Baccalaureus Scientiae Honores-Chemistry, Nelson Mandela Metropolitan University A dissertation submitted in fulfillment of the requirements for the Masters Degree in Chemistry In the Faculty of Science at the NELSON MANDELA METROPOLITAN UNIVERSITY Nov. 2011 Promoter : Dr G. Dugmore Co-Promoter : Prof B. Zeelie 2 DECLARATION I, Lancelot Brooks, hereby declare that the above-mentioned treatise is my own work and that it has not previously been submitted for assessment to another University, or for another qualification. ……………………………….. ……………………….. Mr. L.L. Brooks Date 3 ACKNOWLEDGEMENTS To my promoters Dr. Gary Dugmore, and Prof. Ben Zeelie for their invaluable input, help and guidance. To NRF and NMMU for financial assistance To my parents and brothers for their love and support To Peter, Batsho, Unati, and friends in NMMU chemistry research laboratory, thank you guys. To my dearest fiancée, Natasha, a very special thank you for always being there and supporting me. Love you angel. “And we know that all things work together for good to those who love God, to those who are called according to His purpose” -Romans 8:28. 4 TABLE OF CONTENTS DECLARATION……………………………………………………………………. 2 ACKNOWLEDGEMENTS……………………………………………………………. 3 TABLE OF CONTENTS………………………………………………………………. 4 LIST OF FIGURES…………………………………………………………………….. 8 LIST OF TABLES……………………………………………………………………… 9 LIST OF EQUATIONS………………………………………………………………… 11 SUMMARY……………………………………………………………………………… 12 CHAPTER 1…………………………………………………………………………….. 14 INTRODUCTION………………………………………………………………………. 14 1.1. Technology of leather production……………………………………………….. 14 1.2. Synthesis of TCMTB……………………………………………………………… 17 1.3. Bromine……………………………………………………………………………. 20 1.3.1. Overview……………………………………………………………. 20 1.3.2. Applications of bromine compounds…………..…………………. 22 1.3.2.1. Photography……………………………………………… 22 1.3.2.2. -

Supplement to the Role of Internal Standards and Their Interaction With
Supplement to: Hiatt, M., “The Role of Internal Standards and their Interaction with Soils Impact Accuracy of Volatile Organics Determinations,” Int. J. of Environ. Anal. Chem., 2010 90:8 591-604. Please note that this is a non-copyrighted web version of supplemental information belonging to the above-cited journal article. There are formatting and page-numbering differences between this web version and the actual published version. There are also minor content differences. This version can be accessed at: http://www.epa.gov/nerlesd1/chemistry/vacuum/reference/pubs.htm This publication is also available from Informaworld: http://www.informaworld.com/smpp/content~db=all~content=a921288588 Web version, 27 April 2010 1 Supplement to: The Role of Internal Standards and their Interaction with Soils Impact Accuracy of Volatile Organics Determinations Michael H. Hiatt U.S. Environmental Protection Agency, National Exposure Research Laboratory Environmental Sciences Division. P.O. Box 93478, Las Vegas, Nevada 89193-3478 Phone: 702 798 2381. Fax: 702 798 2142. E-mail: [email protected]. Supplementary Information Data that was too detailed for “The Role of Internal Standards and their Interaction with Soils Impact Accuracy of Volatile Organics Determinations” is presented as supplemental information. This information includes each analyte by matrix and includes the accuracy evaluation, analyte results impacted by criteria, and results from varying spike equilibration times. The list of internal standards and their chemical properties are presented in Table S1 while the surrogates are listed in Table S2. Tables of results by analyte are included as Tables S3-8. These tables include the data by matrix and combined. -

Status of Industry Efforts to Replace Halon Fire Extinguishing Agents
STATUS OF INDUSTRY EFFORTS TO REPLACE HALON FIRE EXTINGUISHING AGENTS Robert T. Wickham, P.E. March 16, 2002 WICKHAM ASSOCIATES NOTICE This document is disseminated under the sponsorship of the U.S. Environmental Protection Agency in the interest of information exchange. The views expressed in this report are those of the author and do not necessarily reflect those of the US Environmental Protection Agency. The US Environmental Protection Agency does not assume liability for the contents or use thereof and further the Agency does not endorse products or manufacturers. Trade or manufacturer's names appear herein solely because they are considered essential to the objective of this report. The following commercial products (requiring a trademark designation ® or ™) are mentioned in this report. Due to the frequency of usage, trademarks are not indicated. Mention of a product does not constitute endorsement or rejection of the product. Ansul FE-25 Argonite FE-36 Argotec FM-200 CEA-410 Halotron I CEA-614 Inergen CEA-308 NAF P-IV Envirogel NAF S-III FE-13 NN100 FE-227 Triodide FE-241 There are no restrictions on copying or distribution of this document. Additional copies of this report are available from ….. Wickham Associates 9 Winding Brook Drive Stratham, New Hampshire 03885 USA Tel: +603-772-3229 Fax: +603-772-7305 email: [email protected] The report is available at http://home.attbi.com/~wickham/downloads.htm for downloading in Adobe Acrobat portable document format (pdf). Preface This report is not intended to be a market study, but instead a snapshot of the progress industry and the government are making in employing non ozone depleting alternatives to halons in the fire protection sector. -
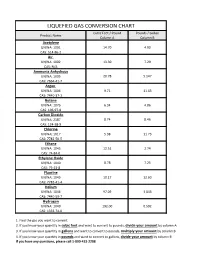
Liquefied Gas Conversion Chart
LIQUEFIED GAS CONVERSION CHART Cubic Feet / Pound Pounds / Gallon Product Name Column A Column B Acetylene UN/NA: 1001 14.70 4.90 CAS: 514-86-2 Air UN/NA: 1002 13.30 7.29 CAS: N/A Ammonia Anhydrous UN/NA: 1005 20.78 5.147 CAS: 7664-41-7 Argon UN/NA: 1006 9.71 11.63 CAS: 7440-37-1 Butane UN/NA: 1075 6.34 4.86 CAS: 106-97-8 Carbon Dioxide UN/NA: 2187 8.74 8.46 CAS: 124-38-9 Chlorine UN/NA: 1017 5.38 11.73 CAS: 7782-50-5 Ethane UN/NA: 1045 12.51 2.74 CAS: 74-84-0 Ethylene Oxide UN/NA: 1040 8.78 7.25 CAS: 75-21-8 Fluorine UN/NA: 1045 10.17 12.60 CAS: 7782-41-4 Helium UN/NA: 1046 97.09 1.043 CAS: 7440-59-7 Hydrogen UN/NA: 1049 192.00 0.592 CAS: 1333-74-0 1. Find the gas you want to convert. 2. If you know your quantity in cubic feet and want to convert to pounds, divide your amount by column A 3. If you know your quantity in gallons and want to convert to pounds, multiply your amount by column B 4. If you know your quantity in pounds and want to convert to gallons, divide your amount by column B If you have any questions, please call 1-800-433-2288 LIQUEFIED GAS CONVERSION CHART Cubic Feet / Pound Pounds / Gallon Product Name Column A Column B Hydrogen Chloride UN/NA: 1050 10.60 8.35 CAS: 7647-01-0 Krypton UN/NA: 1056 4.60 20.15 CAS: 7439-90-9 Methane UN/NA: 1971 23.61 3.55 CAS: 74-82-8 Methyl Bromide UN/NA: 1062 4.03 5.37 CAS: 74-83-9 Neon UN/NA: 1065 19.18 10.07 CAS: 7440-01-9 Mapp Gas UN/NA: 1060 9.20 4.80 CAS: N/A Nitrogen UN/NA: 1066 13.89 6.75 CAS: 7727-37-9 Nitrous Oxide UN/NA: 1070 8.73 6.45 CAS: 10024-97-2 Oxygen UN/NA: 1072 12.05 9.52 CAS: 7782-44-7 Propane UN/NA: 1075 8.45 4.22 CAS: 74-98-6 Sulfur Dioxide UN/NA: 1079 5.94 12.0 CAS: 7446-09-5 Xenon UN/NA: 2036 2.93 25.51 CAS: 7440-63-3 1. -
Ozone Depleting Substances
Ozone Depleting Substances What are they? Ozone depleting substances are chemicals that destroy the earth’s protective ozone layer. They include: • chlorofluorocarbons (CFCs) • halons • carbon tetrachloride (CCl4) • methyl chloroform (CH3CCl3) • hydrobromofluorocarbons (HBFCs) • hydrochlorofluorocarbons (HCFCs) • methyl bromide (CH3Br) • bromochloromethane (CH2BrCl) The use of these chemicals is controlled by the Montreal Protocol on Substances that Deplete the Ozone Layer (the Montreal Protocol). There are other ozone depleting substances, but their ozone depleting effects are very small, so they are not controlled by the Montreal Protocol. One kilogram of halon 1211 can destroy 50 tonnes of ozone What is ozone depleting potential? Ozone depleting potential is a measure of how much damage a chemical can cause to the ozone layer compared with a similar mass What did we use ozone depleting of trichlorofluoromethane (CFC-11). CFC-11, with an ozone depleting potential of 1.0, is substances for? used as the base figure for measuring ozone depletion potential. The higher the number, The main uses of ozone depleting substances the more damage a chemical can cause to include; CFCs and HCFCs in refrigerators and the ozone layer. Bromotrifluoromethane air conditioners, HCFCs and halons in fire (halon -1301) has an ozone depleting potential extinguishers, CFCs and HCFCs in foam, CFCs of 10.0. Carbon dioxide (CO2) is a naturally and HCFCs as aerosol propellants and methyl occurring greenhouse gas, but has an ozone bromide for fumigation of soil, structures and depleting potential of 0. goods to be imported or exported. Have we stopped using ozone Why do we still use some ozone depleting substances? depleting substances? Production of most ozone depleting Some substances with a high ozone depleting substances has been phased out under the potential are still used in quarantine and Montreal Protocol. -

Halons Technical Options Committee 2018 AssessMent Report
Halons Technical Options Committee 2018 Assess ment Report Volume 1 Montreal Protocol on Substances that Deplete the Ozone Layer Ozone Secretariat MONTREAL PROTOCOL ON SUBSTANCES THAT DEPLETE THE OZONE LAYER REPORT OF THE HALONS TECHNICAL OPTIONS COMMITTEE DECEMBER 2018 VOLUME 1 2018 ASSESSMENT REPORT i Montreal Protocol on Substances that Deplete the Ozone Layer Report of the Halons Technical Options Committee December 2018 Volume 1 2018 ASSESSMENT REPORT The text of this report is composed in Times New Roman Co-ordination: Halons Technical Options Committee Composition of the report: Halons Technical Options Committee Reproduction: Ozone Secretariat Date: December 2018 Under certain conditions, printed copies of this report are available from: UNITED NATIONS ENVIRONMENT PROGRAMME Ozone Secretariat, P.O. Box 30552, Nairobi, Kenya This document is also available in portable document format from the Ozone Secretariat's website: https://ozone.unep.org/sites/default/files/Assessment_Panel/Assessment_Panels/TEAP/R eports/HTOC/HTOC_assessment_2018.pdf No copyright involved. This publication may be freely copied, abstracted and cited, with acknowledgement of the source of the material. ISBN: 978-9966-076-48-9 iii Disclaimer The United Nations Environment Programme (UNEP), the Technology and Economic Assessment Panel (TEAP) Co-chairs and members, the Technical Options Committees Co-chairs and members, the TEAP Task Forces Co-chairs and members, and the companies and organisations that employ them do not endorse the performance, worker safety, or environmental acceptability of any of the technical options discussed. Every industrial operation requires consideration of worker safety and proper disposal of contaminants and waste products. Moreover, as work continues - including additional toxicity evaluation - more information on health, environmental and safety effects of alternatives and replacements will become available for use in selecting among the options discussed in this document. -

SROC Annex V
Annex V Major Chemical Formulae and Nomenclature This annex presents the formulae and nomenclature for halogen-containing species and other species that are referred to in this report (Annex V.1). The nomenclature for refrigerants and refrigerant blends is given in Annex V.2. V.1 Substances by Groupings V.1.1 Halogen-Containing Species V.1.1.1 Inorganic Halogen-Containing Species Atomic chlorine Cl Atomic bromine Br Molecular chlorine Cl2 Molecular bromine Br2 Chlorine monoxide ClO Bromine monoxide BrO Chlorine radicals ClOx Bromine radicals BrOx Chloroperoxy radical ClOO Bromine nitrate BrONO2, BrNO3 Dichlorine peroxide (ClO dimer) (ClO)2, Cl2O2 Potassium bromide KBr Hydrogen chloride (Hydrochloric acid) HCl Inorganic chlorine Cly Antimony pentachloride SbCl5 Atomic fluorine F Molecular fluorine F2 Atomic iodine I Hydrogen fluoride (Hydrofluoric acid) HF Molecular iodine I2 Sulphur hexafluoride SF6 Nitrogen trifluoride NF3 IPCC Boek (dik).indb 467 15-08-2005 10:57:13 468 IPCC/TEAP Special Report: Safeguarding the Ozone Layer and the Global Climate System V.1.1.2 Halocarbons For each halocarbon the following information is given in columns: • Chemical compound [Number of isomers]1 (or common name) • Chemical formula • CAS number2 • Chemical name (or alternative name) V.1.1.2.1 Chlorofluorocarbons (CFCs) CFC-11 CCl3F 75-69-4 Trichlorofluoromethane CFC-12 CCl2F2 75-71-8 Dichlorodifluoromethane CFC-13 CClF3 75-72-9 Chlorotrifluoromethane CFC-113 [2] C2Cl3F3 Trichlorotrifluoroethane CCl FCClF 76-13-1 CFC-113 2 2 1,1,2-Trichloro-1,2,2-trifluoroethane -

ACE Tips for Filing EPA ODS
Automated Commercial Environment ACE Tips for Filing EPA ODS June, 2018 Ozone-depleting substances (ODS) deplete the stratospheric ozone layer when the chlorine and bromine atoms that they contain come into contact with ozone molecules. One chlorine atom can destroy over 100,000 ozone molecules before it is removed from the stratosphere. ODS that release chlorine include chlorofluorocarbons (CFCs), hydrochlorofluorocarbons (HCFCs), carbon tetrachloride, and methyl chloroform. ODS that release bromine include halons and methyl bromide. HTS code Ozone-Depleting Substance 2903.14.0000 Carbon tetrachloride What commodities 2903.19.6010 Methylchloroform (1,1,1-Trichloroethane) are ODS used in? 2903.39.1520 Methyl bromide 2903.71.0000 Chlorodifluoromethane (HCFC-22) ODS have been used 2903.72.0020 Dichlorotrifluoroethane (HCFC-123) for refrigeration, air 2903.73.0000 Dichlorofluoroethanes (HCFC-141, 141b) conditioning, insulation, 2903.74.0000 Chlorodifluoroethanes (HCFC-142, 142b) solvents, aerosol 2903.75.0000 Dichloropentafluoropropanes propellants, and in other (HCFC-225, 225ca, 225cb) sectors. Currently, the United 2903.76.0010 Bromotrifluoromethane (Halon 1301) States is in the 2903.76.0050 Bromochlorodifluoromethane (Halon 1211), process of phasing bromotrifluoromethane (Halon 1301), and out HCFCs, but in the dibromotetrafluoroethanes (Halon 2402), Other interim limited imports are 2903.77.0010 Trichlorofluoromethane (CFC-11) allowed. CFCs, 2903.77.0020 Trichlorotrifluoroethanes (CFC-113, CFC-113a) methyl bromide, halon, 2903.77.0030 -

List of Lists
United States Office of Solid Waste EPA 550-B-10-001 Environmental Protection and Emergency Response May 2010 Agency www.epa.gov/emergencies LIST OF LISTS Consolidated List of Chemicals Subject to the Emergency Planning and Community Right- To-Know Act (EPCRA), Comprehensive Environmental Response, Compensation and Liability Act (CERCLA) and Section 112(r) of the Clean Air Act • EPCRA Section 302 Extremely Hazardous Substances • CERCLA Hazardous Substances • EPCRA Section 313 Toxic Chemicals • CAA 112(r) Regulated Chemicals For Accidental Release Prevention Office of Emergency Management This page intentionally left blank. TABLE OF CONTENTS Page Introduction................................................................................................................................................ i List of Lists – Conslidated List of Chemicals (by CAS #) Subject to the Emergency Planning and Community Right-to-Know Act (EPCRA), Comprehensive Environmental Response, Compensation and Liability Act (CERCLA) and Section 112(r) of the Clean Air Act ................................................. 1 Appendix A: Alphabetical Listing of Consolidated List ..................................................................... A-1 Appendix B: Radionuclides Listed Under CERCLA .......................................................................... B-1 Appendix C: RCRA Waste Streams and Unlisted Hazardous Wastes................................................ C-1 This page intentionally left blank. LIST OF LISTS Consolidated List of Chemicals -

Maine Remedial Action Guidelines (Rags) for Contaminated Sites
Maine Department of Environmental Protection Remedial Action Guidelines for Contaminated Sites (RAGs) Effective Date: May 1, 2021 Approved by: ___________________________ Date: April 27, 2021 David Burns, Director Bureau of Remediation & Waste Management Executive Summary MAINE DEPARTMENT OF ENVIRONMENTAL PROTECTION 17 State House Station | Augusta, Maine 04333-0017 www.maine.gov/dep Maine Department of Environmental Protection Remedial Action Guidelines for Contaminated Sites Contents 1 Disclaimer ...................................................................................................................... 1 2 Introduction and Purpose ............................................................................................... 1 2.1 Purpose ......................................................................................................................................... 1 2.2 Consistency with Superfund Risk Assessment .............................................................................. 1 2.3 When to Use RAGs and When to Develop a Site-Specific Risk Assessment ................................. 1 3 Applicability ................................................................................................................... 2 3.1 Applicable Programs & DEP Approval Process ............................................................................. 2 3.1.1 Uncontrolled Hazardous Substance Sites ............................................................................. 2 3.1.2 Voluntary Response Action Program -

1 HUMAN HEALTH SAFETY EVALUATION of HALON REPLACEMENT CANDIDATES Darol E. Dodd1, Gary W. Jepson1, and Joseph A. Macko, Jr.2 1Air
HUMAN HEALTH SAFETY EVALUATION OF HALON REPLACEMENT CANDIDATES Darol E. Dodd1, Gary W. Jepson1, and Joseph A. Macko, Jr.2 1Air Force Research Laboratory/Operational Toxicology Branch (AFRL/HEST) – ManTech Environmental Technology, Inc. Wright-Patterson Air Force Base, Ohio 2Toxicology Directorate (MCHB-TS-T)/U.S. Army Center for Health Promotion & Preventive Medicine Aberdeen Proving Ground, Maryland Next-Generation Fire Suppression Technology Program (NGP) Project 3B/1/89 Final Report – Section II of II This research is part of the Department of Defense’s Next-Generation Fire Suppression Technology Program, funded by the DoD Strategic Environmental Research and Development Program (SERDP) 1 TABLE OF CONTENTS Page INTRODUCTION……………………………...………………………………………………….4 PHASE 1 – TOXICITY SCREENING METHODS……………………...……………………….5 Chemical/physical properties……………………………………………….…………….6 Existing toxicity literature…………………………………………………..…………….6 Preliminary “specific use” scenarios………………………………………..…………….7 Qualitative/quantitative structure activity relationships……………………..……………7 In vitro screens……………………………………………………………...…………….8 Acute irritation tests………………………………………………………………………9 Acute toxicity tests…………………………………………………………..…………..10 The “limit test”…………………………………………………………………..11 The “specific use” scenario test……………………………………..…………..12 Genotoxicity tests (Phase 1)………………………………………………..……………13 DECISION POINT 1……………………………………………………………………14 Concentration x time (C x T) relationships……………………….……………18 Stop or continue testing…………………………………………..…………….18 PHASE 2 – TOXICITY