Conifers Response to Water Stress: Physiological Responses and Effects on Nutrient Use Physiology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Role of Fir Species in the Silviculture of British Forests
Kastamonu Üni., Orman Fakültesi Dergisi, 2012, Özel Sayı: 15-26 Kastamonu Univ., Journal of Forestry Faculty, 2012, Special Issue The Role of True Fir Species in the Silviculture of British Forests: past, present and future W.L. MASON Forest Research, Northern Research Station, Roslin, Midlothian, Scotland EH25 9SY, U.K. E.mail:[email protected] Abstract There are no true fir species (Abies spp.) native to the British Isles: the first to be introduced was Abies alba in the 1600s which was planted on some scale until the late 1800s when it proved vulnerable to an insect pest. Thereafter interest switched to North American species, particularly grand (Abies grandis) and noble (Abies procera) firs. Provenance tests were established for A. alba, A. amabilis, A. grandis, and A. procera. Other silver fir species were trialled in forest plots with varying success. Although species such as grand fir have proved highly productive on favourable sites, their initial slow growth on new planting sites and limited tolerance of the moist nutrient-poor soils characteristic of upland Britain restricted their use in the afforestation programmes of the last century. As a consequence, in 2010, there were about 8000 ha of Abies species in Britain, comprising less than one per cent of the forest area. Recent species trials have confirmed that best growth is on mineral soils and that, in open ground conditions, establishment takes longer than for other conifers. However, changes in forest policies increasingly favour the use of Continuous Cover Forestry and the shade tolerant nature of many fir species makes them candidates for use with selection or shelterwood silvicultural systems. -

40YEARS 1980-2020 Check out Our Plugs
Brooks Tree Farm CELEBRATING 40YEARS 1980-2020 Check out our Plugs Norway Spruce is grown in much of the USA, it tolerates a wide range of soils and takes shearing well. Good cold climate Christmas tree, also used as understock for all Spruce grafting. Colorado Spruce has a wide variety of colors, some more blue than others. Native to the Rocky Mountains, but used all over as an ornamental species. Needles can be sharp to the touch. Ponderosa Pine grows very straight and tall and has long needles. Norway Spruce Colorado Spruce Ponderosa Pine The Willamette Valley seed source was nearly extinct, it tolerates wet soil. Mugo Mughus is a slow growing pine that reaches 8’. Ornamentally used in landscapes, embankments, rock walls, Mughus is very resistant to cold and drought. Salal is a low ornamental shrub. The leaves are often used by florists. Native to the west coast Salal is often found on Giantembankments Sequoia on is the a fast coast growing or on forest conifer floors. to 200’ from California, often grafted with weeping form. Also popular as large scale evergreen screen. Mugo Mughus Salal Giant Sequoia Root Dip Protect your investment by dipping roots in a super absorbent root protecting hydrogel prior Horta Sorb to planting. The gels attract moisture and hold it in the root zone keeping it accessible to the 1 Pound $25.00 roots during dry periods. The polymer gels can absorb hundreds of times their weight in 4 Pounds $80.00 water and release the moisture to the plant multiple times, providing water to the root zone 10 Pounds $150.00 and protecting the plants during roots establishment. -

Proceedings of the 9Th International Christmas Tree Research & Extension Conference
Proceedings of the 9th International Christmas Tree Research & Extension Conference September 13–18, 2009 _________________________________________________________________________________________________________ John Hart, Chal Landgren, and Gary Chastagner (eds.) Title Proceedings of the 9th International Christmas Tree Research & Extension Conference IUFRO Working Unit 2.02.09—Christmas Trees Corvallis, Oregon and Puyallup, Washington, September 13–18, 2009 Held by Oregon State University, Washington State University, and Pacific Northwest Christmas Tree Growers’ Association Editors John Hart Chal Landgren Gary Chastagner Compilation by Teresa Welch, Wild Iris Communications, Corvallis, OR Citation Hart, J., Landgren, C., and Chastagner, G. (eds.). 2010. Proceedings of the 9th International Christmas Tree Research and Extension Conference. Corvallis, OR and Puyallup, WA. Fair use This publication may be reproduced or used in its entirety for noncommercial purposes. Foreword The 9th International Christmas Tree Research and Extension Conference returned to the Pacific Northwest in 2009. OSU and WSU cohosted the conference, which was attended by 42 Christmas tree professionals representing most of the major production areas in North America and Europe. This conference was the most recent in the following sequence: Date Host Location Country October 1987 Washington State University Puyallup, Washington USA August 1989 Oregon State University Corvallis, Oregon USA October 1992 Oregon State University Silver Falls, Oregon USA September 1997 -

Wa Shan – Emei Shan, a Further Comparison
photograph © Zhang Lin A rare view of Wa Shan almost minus its shroud of mist, viewed from the Abies fabri forested slopes of Emei Shan. At its far left the mist-filled Dadu River gorge drops to 500-600m. To its right the 3048m high peak of Mao Kou Shan climbed by Ernest Wilson on 3 July 1903. “As seen from the top of Mount Omei, it resembles a huge Noah’s Ark, broadside on, perched high up amongst the clouds” (Wilson 1913, describing Wa Shan floating in the proverbial ‘sea of clouds’). Wa Shan – Emei Shan, a further comparison CHRIS CALLAGHAN of the Australian Bicentennial Arboretum 72 updates his woody plants comparison of Wa Shan and its sister mountain, World Heritage-listed Emei Shan, finding Wa Shan to be deserving of recognition as one of the planet’s top hotspots for biological diversity. The founding fathers of modern day botany in China all trained at western institutions in Europe and America during the early decades of last century. In particular, a number of these eminent Chinese botanists, Qian Songshu (Prof. S. S. Chien), Hu Xiansu (Dr H. H. Hu of Metasequoia fame), Chen Huanyong (Prof. W. Y. Chun, lead author of Cathaya argyrophylla), Zhong Xinxuan (Prof. H. H. Chung) and Prof. Yung Chen, undertook their training at various institutions at Harvard University between 1916 and 1926 before returning home to estab- lish the initial Chinese botanical research institutions, initiate botanical exploration and create the earliest botanical gardens of China (Li 1944). It is not too much to expect that at least some of them would have had personal encounters with Ernest ‘Chinese’ Wilson who was stationed at the Arnold Arboretum of Harvard between 1910 and 1930 for the final 20 years of his life. -
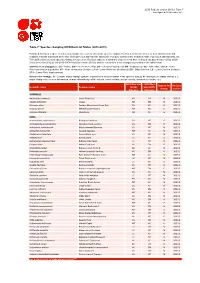
Table 7: Species Changing IUCN Red List Status (2012-2013)
IUCN Red List version 2013.2: Table 7 Last Updated: 25 November 2013 Table 7: Species changing IUCN Red List Status (2012-2013) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2012 (IUCN Red List version 2012.2) and 2013 (IUCN Red List version 2013.2) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered, EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.) IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2012) List (2013) change version Category Category MAMMALS Nycticebus javanicus Javan Slow Loris EN CR N 2013.2 Okapia johnstoni Okapi NT EN N 2013.2 Pteropus niger Greater Mascarene Flying -

The Evolution of Cavitation Resistance in Conifers Maximilian Larter
The evolution of cavitation resistance in conifers Maximilian Larter To cite this version: Maximilian Larter. The evolution of cavitation resistance in conifers. Bioclimatology. Univer- sit´ede Bordeaux, 2016. English. <NNT : 2016BORD0103>. <tel-01375936> HAL Id: tel-01375936 https://tel.archives-ouvertes.fr/tel-01375936 Submitted on 3 Oct 2016 HAL is a multi-disciplinary open access L'archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destin´eeau d´ep^otet `ala diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publi´esou non, lished or not. The documents may come from ´emanant des ´etablissements d'enseignement et de teaching and research institutions in France or recherche fran¸caisou ´etrangers,des laboratoires abroad, or from public or private research centers. publics ou priv´es. THESE Pour obtenir le grade de DOCTEUR DE L’UNIVERSITE DE BORDEAUX Spécialité : Ecologie évolutive, fonctionnelle et des communautés Ecole doctorale: Sciences et Environnements Evolution de la résistance à la cavitation chez les conifères The evolution of cavitation resistance in conifers Maximilian LARTER Directeur : Sylvain DELZON (DR INRA) Co-Directeur : Jean-Christophe DOMEC (Professeur, BSA) Soutenue le 22/07/2016 Devant le jury composé de : Rapporteurs : Mme Amy ZANNE, Prof., George Washington University Mr Jordi MARTINEZ VILALTA, Prof., Universitat Autonoma de Barcelona Examinateurs : Mme Lisa WINGATE, CR INRA, UMR ISPA, Bordeaux Mr Jérôme CHAVE, DR CNRS, UMR EDB, Toulouse i ii Abstract Title: The evolution of cavitation resistance in conifers Abstract Forests worldwide are at increased risk of widespread mortality due to intense drought under current and future climate change. -

Supplementary Table S2 Details of 455 Conifer Species Used in the Phylogene�C and Physiological Niche Modelling to Es�Mate Drivers of Diversifica�On
Supplementary Table S2 Details of 455 conifer species used in the phylogene�c and physiological niche modelling to es�mate drivers of diversifica�on. Shown are: the clade calcifica�on (10 and 42 clade); number of cleaned georeferenced presence records; the confusion matrix which describes the model fit in terms of true posi�ves, true nega�ves, false posi�ves and false nega�ves; and the es�mated niche area in quarter degree grid squares for the globe (projected) and for version of the globe where all environmental zones are equally common (resampled), see main text for further details. Clade classifica�on Confusion matrix niche area (# grid cells) 42 (68*) Number of True True False False Species 10 clades clades records posi�ves nega�ves posi�ves nega�ves Projected Resampled Abies alba 10 65 119 117 111 4 2 6658 7622 Abies amabilis 10 65 80 79 74 2 0 11783 13701 Abies bracteata 10 65 4 4 15 0 0 1610 1846 Abies concolor 10 65 98 90 86 8 8 13825 15410 Abies fabri 10 65 4 4 17 0 0 2559 2641 Abies fargesii 10 65 13 13 18 0 0 14450 15305 Abies firma 10 65 163 161 163 1 0 2270 2436 Abies fraseri 10 65 15 15 16 0 0 1914 2075 Abies grandis 10 65 77 75 70 2 2 11654 13629 Abies holophylla 10 65 12 12 16 1 0 23899 24592 Abies homolepis 10 65 31 31 34 0 0 791 851 Abies kawakamii 10 65 17 17 26 0 0 700 1164 Abies koreana 10 65 10 10 18 0 0 985 1048 Abies lasiocarpa 10 65 105 100 95 6 5 11422 12454 Abies magnifica 10 65 47 47 58 2 0 11882 14353 Abies mariesii 10 65 16 16 17 0 0 3833 4114 Abies nebrodensis 10 65 1 1 17 0 0 1094 973 Abies nephrolepis 10 65 -

Erfahrungen Mit Abies-Arten in Südwestdeutschland Von
Erfahrungen mit Abies-Arten in Südwestdeutschland Von HUBERTUS NIMSCH Zusammenfassung In den vergangenen Jahrzehnten sind vom Autor Kulturversuche im Arboretum Günterstal, Freiburg, mit über 60 Tannen-Arten und Varietäten aus aller Welt durchgeführt worden. Hier wird in alphabetischer Folge über deren natürliche Verbreitung, die genetische und taxonomische Differenzierung sowie über die bisherigen Kultur-Erfahrungen unter südwestdeutschen Klimabedingungen berichtet. Einführung Eine intensive praktische Beschäftigung mit zahlreichen, z.T. selten kultivierten Arten der Gattung Abies (die zur Familie der Pinaceae, Unterfamilie Abietoideae, gehört) ist der Anlass diese über einen Zeitraum von mehreren Jahrzehnten gesammelten Erfahrungen in knappen Worten zusammen zu tragen. Diese Erfahrungen aus dem Arboretum Freiburg-Günterstal können und sollen nur stichwortartig dargestellt werden. Ebenso stichwortartig soll versucht werden, die jeweilige Abies-Art abrundend mit ihrem korrekten wissenschaftlichen Namen, ihren Synonyma sowie mit ihrem deutschen und englischen Namen (soweit vorhanden) und dem einheimischen Namen darzustellen. Kurz gefasst werden einige Bemerkungen zu den Themen Naturvorkommen, genetische Differenzierung, weiterführende Literatur bzw. und Ökologie gemacht. Zum Thema „Örtliche Erfahrungen“ folgen Aussagen, die sich nur auf den Bereich Freiburg und den Westabfall des Schwarzwaldes beziehen. Erfahrungen an anderen Standorten können deshalb durchaus verschieden oder widersprüchlich sein. Bewusst wird auf eine allgemeine Beschreibung der Tannenarten, auf Standort – und Klimaansprüche, auf Wachstum und Entwicklung, auf Nutzung und Pathologie u.a. verzichtet und stattdessen auf weiterführende Literatur bzw. auf Autorennamen verwiesen. Aus der Reihenfolge der im Schrifttum genannten Autoren ist keine Wertigkeit abzuleiten. Neben der umfassenden Abies-Monographie von LIU (1971) wurde bezüglich der chinesischen Tannenarten den Aussagen von CHENG (1978) und bezüglich der mexikanischen Tannenarten den Aussagen von MARTINEZ (1963) größere Bedeutung beigemessen. -

Genetic Differentiation of Abies Equi-Trojani (Asch. & Sint. Ex Boiss
Turk J Bot 32 (2008) 1-10 © TÜB‹TAK Research Article Genetic Differentiation of Abies equi-trojani (Asch. & Sint. ex Boiss) Mattf. Populations from Kazda¤›, Turkey and the Genetic Relationship between Turkish Firs belonging to the Abies nordmanniana Spach Complex Zeki KAYA1*, A. SKAGGS2, David Brian NEALE2,3 1 Department of Biological Sciences, Middle East Technical University, 06531 Ankara - TURKEY 2 Institute of Forest Genetics, USDA Forest Service, Pacific Southwest Research Station, 2480 Carson Rd., Placerville CA 95667 USA 3 Department of Plant Sciences , University of California at Davis, One Shields Avenue, Davis, CA 95616 USA Received: 05.02.2006 Accepted: 15.11.2007 Abstract: The present study aimed to test the utility of RAPD (randomly amplified polymorphic DNA) and cpSSR (simple sequence repeats) markers for in situ gene conservation programs for fir species, as well as for determining the genetic similarities between the Abies nordmanniana Spach species complex (A. nordmanniana, A. bornmuelleriana Matff., A. equi-trojani (Asch. & Sint. ex Boiss) Mattf.) and between populations of A. equi-trojani, which is a narrow-endemic to Turkey. For this purpose, DNA was extracted and pooled from 15 seed megagametophytes (megs) of the Ortaköy population of A. nordmanniana and the Muratdere population of A. bornmuelleriana species, and from two 7-meg subsamples each from of the Kazda¤› and Çan populations of A. equi-trojani. Template DNA was screened with the DNA markers to reveal the amount of genetic variation in each species. It appeared that template DNA pooling for screening the fir populations with RAPD or cp-SSR markers could be effectively used to speed up gene conservation and taxonomic studies. -

Division: SPERMATOPHYTA (Flowering Plants)
Division: SPERMATOPHYTA (Flowering plants) Most important features of plants in Spermtophyta is having flowers and seeds. - Anthophyta (Flowering plants) - Spermatophyta (Plants with seeds) Male gametophyte has become polen grain, and female gametophyte has become embryo vesicle. The seed and the embryo are covered with a special seed coat and can wait for germination until optimal conditions form. Polen grains reach the ovule via wind (anemogamous plants) or bugs (entemogamous plants), etc. Plants are divided into two subclasses as Gymnospermae and Angiospermae according to their ability to form a closed ovarium, or not. anth(o)= Gr. flower; sperm(ato) = Gr. seed gymn(o)= Gr. naked; angio= Gr. narrow (closed) Subdivision: GYMNOSPERMAE (Plants with naked seeds) An ovarium protecting the ovules is not present. Therefore, stylus and stigma are also absent. The micropyle is open and the polen grains enter directly into the polen room found in the ovule and germinate there. Fertilization occurs via the wind, so Gymnospermae plants are anemogamous plants. Gymnospermae plants are in the form of shrubs and trees; herbaceous species are not found among them. Their flowers lack calyx and corolla; male flowers are reduced to polen vesicles and female flowers are reduced to ovules. Both male and female inflorescences are in the form of cones (strobiles). Pollination is via the wind Class: Cycadinae Fam: Cycadaceae Grows in tropical or subtropical regions. Cycas revoluta (King sago, Sago cycad, sikas) A starch called Sago Starch is obtained from this plant and used as food. Class: Ginkgoiae Fam: Ginkgoaceae This family dates back to geological periods, however today it is presented with a single genus and a single species. -

Use and Transfer of Forest Reproductive Material in Europe in the Context of Climate Change
EUROPEAN FOREST GENETIC RESOURCES PROGRAMME Use and transfer of forest EUFORGEN reproductive material in Europe in the context of climate change Monika Konnert, Bruno Fady, Dusˇ an Gömöry, Stuart A’Hara, Frank Wolter, Fulvio Ducci, Jarkko Koskela, Michele Bozzano, Tiit Maaten and Jan Kowalczyk U SE AND T RANSFER OF FR M Bioversity International is a global research-for-development organization. We have a vision – that ag- ricultural biodiversity nourishes people and sustains the planet. We deliver scientific evidence, manage- ment practices and policy options to use and safeguard agricultural and tree biodiversity to attain sus- tainable global food and nutrition security. We work with partners in low-income countries in different regions where agricultural and tree biodiversity can contribute to improved nutrition, resilience, produc- tivity and climate change adaptation. Bioversity International is a member of the CGIAR Consortium – a global research partnership for a food-secure future. European Forest Genetic Resources Programme (EUFORGEN) is an instrument of international co- operation promoting the conservation and appropriate use of forest genetic resources in Europe. It was established in 1994 to implement Strasbourg Resolution 2 adopted by the first Ministerial Conference of the FOREST EUROPE process, held in France in 1990. EUFORGEN also contributes to implementa- tion of other FOREST EUROPE commitments on forest genetic resources and relevant decisions of the Convention on Biological Diversity (CBD). Furthermore, EUFORGEN contributes to the implementation of regional-level strategic priorities of the Global Plan of Action for the Conservation, Sustainable Use and Development of Forest Genetic Resources (GPA-FGR), adopted by the FAO Conference in 2013. -
Download Royal Botanic Garden Edinburgh Collection Policy for The
Collection Policy for the Living Collection David Rae (Editor), Peter Baxter, David Knott, David Mitchell, David Paterson and Barry Unwin 3908_Coll_policy.indd 1 21/9/06 3:24:25 pm 2 | ROYAL BOTANIC GARDEN EDINBURGH COLLECTION POLICY FOR THE LIVING COLLECTION Contents Regius Keeper’s Foreword 3 Section IV Collection types 22 Introduction 22 Introduction 3 Conservation collections 22 PlantNetwork Target 8 project 24 Purpose, aims and objectives 3 Scottish Plant Project 25 International Conifer Conservation Programme 25 Section I National and international context, National Council for the Conservation of Plants stakeholders and user groups 4 and Gardens (NCCPG) 25 Off-site collections 26 National and international context 4 Convention on Biological Diversity 4 Heritage or historic plants, plant collections or Global Strategy for Plant Conservation 4 landscape features 27 Plant Diversity Challenge 5 British native plants International Agenda for Botanic Gardens in Scottish Plants Project (formerly known as the Conservation 6 Scottish Rare Plants Project) 29 Action Plan for the Botanic gardens in the European Union & Planta Europa 6 Section V Acquisition and transfer 30 Convention on Trade in Endangered species of Introduction 30 Wild Flora and Fauna 8 Fieldwork 30 Global warming/outside influences 8 Index Seminum 31 Stakeholders and user groups 9 Other seed and plant catalogues 31 Research and conservation 9 Acquisition 31 Education and teaching 10 Repatriation 32 Interpretation 10 Policy for the short to medium term storage Phenology 10