Panels, Mini-Panels and Study Groupsdecember 4
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Activation of the Glucagon-Like Peptide-1 (GLP-1) Receptor by Peptide and Non-Peptide Ligands
The Activation of the Glucagon-Like Peptide-1 (GLP-1) Receptor by Peptide and Non-Peptide Ligands Clare Louise Wishart Submitted in accordance with the requirements for the degree of Doctor of Philosophy of Science University of Leeds School of Biomedical Sciences Faculty of Biological Sciences September 2013 I Intellectual Property and Publication Statements The candidate confirms that the work submitted is her own and that appropriate credit has been given where reference has been made to the work of others. This copy has been supplied on the understanding that it is copyright material and that no quotation from the thesis may be published without proper acknowledgement. The right of Clare Louise Wishart to be identified as Author of this work has been asserted by her in accordance with the Copyright, Designs and Patents Act 1988. © 2013 The University of Leeds and Clare Louise Wishart. II Acknowledgments Firstly I would like to offer my sincerest thanks and gratitude to my supervisor, Dr. Dan Donnelly, who has been nothing but encouraging and engaging from day one. I have thoroughly enjoyed every moment of working alongside him and learning from his guidance and wisdom. My thanks go to my academic assessor Professor Paul Milner whom I have known for several years, and during my time at the University of Leeds he has offered me invaluable advice and inspiration. Additionally I would like to thank my academic project advisor Dr. Michael Harrison for his friendship, help and advice. I would like to thank Dr. Rosalind Mann and Dr. Elsayed Nasr for welcoming me into the lab as a new PhD student and sharing their experimental techniques with me, these techniques have helped me no end in my time as a research student. -
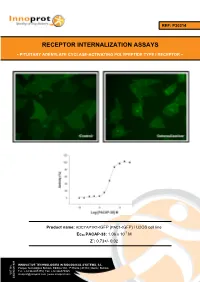
Receptor Internalization Assays
REF: P30214 RECEPTOR INTERNALIZATION ASSAYS - PITUITARY ADENYLATE CYCLASE-ACTIVATING POLYPEPTIDE TYPE I RECEPTOR - Product name: ADCYAP1R1-tGFP (PAC1-tGFP) / U2OS cell line -7 Ec50 PACAP-38: 1.06 x 10 M Z´: 0.73+/- 0.02 INNOVATIVE TECHNOLOGIES IN BIOLOGICAL SYSTEMS, S.L. Parque Tecnológico Bizkaia, Edifício 502, 1ª Planta | 48160 | Derio | Bizkaia Tel.: +34 944005355 | Fax: +34 946579925 VAT No. [email protected] | www.innoprot.com ESB95481909 Product Name: ADCYAP1R1-tGFP_U2OS Reference: P30214 Rep. Official Full Name: Pituitary adenylate cyclase- activating polypeptide type I receptor DNA Accession Number: Gene Bank AY366498 Host Cell: U2OS References: P30214: 2 vials of 3 x 106 proliferative cells P30214-DA: 1 vial of 2 x 106 division-arrested cells Storage: Liquid Nitrogen Assay Briefly description About ADCYAP1R1 Each vial of ADCYAP1R1 Internalization Assay Pituitary adenylate cyclase-activating Cell Line contains U2OS cells stably expressing polypeptide type I receptor, also known as human Pituitary adenylate cyclase-activating PAC1 is a protein that in humans is encoded by polypeptide type I receptor tagged in the N- the ADCYAP1R1 gene. ADCYAP1R1 is a terminus with tGFP protein. membrane-associated protein and shares significant homology with members of the Innoprot’s ADCYAP1R1-tGFP Internalization glucagon/secretin receptor family. This receptor Assay Cell Line has been designed to assay binds pituitary adenylate cyclase activating potential agonists/ antagonists against peptide (PACAP) mediating several biological ADCYAP1R1, modulating its activation and the activities and it is positively coupled to following redistribution process inside the cells. adenylate cyclase. This cell line will allow the image analysis of the stimuli induced by the compounds. -

Debbie Sookman Is an Outstanding Contribution to the Science and Clinical Practice Related to the Full Range of Obsessive Com- Pulsive Disorder
Downloaded by [New York University] at 04:59 12 August 2016 “I strongly recommend this expert clinical guide to the psychological treat- ment of obsessive compulsive disorders. The depth of Dr. Sookman’s clinical experience and her command of the literature are evident in the thorough coverage of assessment procedures, how to optimize the effects of therapy and deal with problems. The numerous case illustrations are well-chosen and clearly described.” —S. Rachman, Emeritus Professor, Institute of Psychiatry, London University, and University of British Columbia. “Specialized Cognitive Behavior Therapy for Obsessive Compulsive Disorder: An Expert Clinician Guidebook by Dr. Debbie Sookman is an outstanding contribution to the science and clinical practice related to the full range of Obsessive Com- pulsive Disorder. This is an excellent book in every way imaginable. Clearly written and organized, Sookman provides a critical and scholarly review of the state of the art on OCD. Every researcher and clinician can benefit from this superb book. The reader benefits from the considerable clinical expe- rience and scholarship that Dr Sookman possesses, while learning specific and powerful tools in helping those who suffer from OCD. Case examples illustrate the importance of conceptualization and the value of empirically supported treatments. I am particularly impressed that Sookman was able to balance such sophistication in her critical and scientific understanding of OCD, while still writing a clear and concise book on the topic. This is a book I will recommend to both beginning clinicians in training and to seasoned researchers and practitioners.” —Robert L. Leahy, Ph.D., Director, American Institute for Cognitive Therapy “Dr. -

Novel Gene Fusions in Glioblastoma Tumor Tissue and Matched Patient Plasma
cancers Article Novel Gene Fusions in Glioblastoma Tumor Tissue and Matched Patient Plasma 1, 1, 1 1 1 Lan Wang y, Anudeep Yekula y, Koushik Muralidharan , Julia L. Small , Zachary S. Rosh , Keiko M. Kang 1,2, Bob S. Carter 1,* and Leonora Balaj 1,* 1 Department of Neurosurgery, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02115, USA; [email protected] (L.W.); [email protected] (A.Y.); [email protected] (K.M.); [email protected] (J.L.S.); [email protected] (Z.S.R.); [email protected] (K.M.K.) 2 School of Medicine, University of California San Diego, San Diego, CA 92092, USA * Correspondence: [email protected] (B.S.C.); [email protected] (L.B.) These authors contributed equally. y Received: 11 March 2020; Accepted: 7 May 2020; Published: 13 May 2020 Abstract: Sequencing studies have provided novel insights into the heterogeneous molecular landscape of glioblastoma (GBM), unveiling a subset of patients with gene fusions. Tissue biopsy is highly invasive, limited by sampling frequency and incompletely representative of intra-tumor heterogeneity. Extracellular vesicle-based liquid biopsy provides a minimally invasive alternative to diagnose and monitor tumor-specific molecular aberrations in patient biofluids. Here, we used targeted RNA sequencing to screen GBM tissue and the matched plasma of patients (n = 9) for RNA fusion transcripts. We identified two novel fusion transcripts in GBM tissue and five novel fusions in the matched plasma of GBM patients. The fusion transcripts FGFR3-TACC3 and VTI1A-TCF7L2 were detected in both tissue and matched plasma. -

RAP-MD-05 GLYX13-C-203 Protocol Amendment 3 Final-R
NCT02192099 Study ID: GLYX13‐C‐203 Title: Open Label Extension for Subjects with Inadequate/Partial Response to Antidepressants during the Current Episode of Major Depressive Disorder Previously Treated with Rapastinel (GLYX‐13) (Extension of GLYX13‐C‐202, NCT01684163) Protocol Amendment 3 Date: 23 Nov 2016 Clinical Study Protocol Protocol Number: GLYX13-C-203 Study Drug: Rapastinel (GLYX-13) Injection (rapastinel Injection) FDA ID: 107,974 Clinicaltrials.gov: Title: Open Label Extension for Subjects with Inadequate/Partial Response to Antidepressants during the Current Episode of Major Depressive Disorder Previously Treated with Rapastinel (GLYX-13) (Extension of GLYX13-C-202, NCT01684163) Study Phase: 2 Sponsor: Naurex, Inc, an affiliate of Allergan, plc. Date: Amendment 3 dated 23 November 2016 Replaces Amendment 2 dated 20 January 2015 This document is a confidential communication from Naurex, Inc, an affiliate of Allergan, plc.. Acceptance of this document constitutes an agreement by the recipient(s) that no information contained herein will be published or disclosed without prior written approval from Allergan, plc., except that this document may be disclosed to appropriate Institutional Review Boards (IRBs) under the condition that they are also required to maintain confidentiality. Naurex, Inc, an affiliate of Allergan, plc. Protocol GLYX13-C-203 INVESTIGATOR SIGNATURE PAGE The signature of the investigator below constitutes his/her approval of this protocol and provides the necessary assurances that this study will be conducted according to all stipulations of the protocol as specified in both the clinical and administrative sections, including all statements regarding confidentiality. This trial will be conducted in compliance with the protocol and all applicable regulatory requirements, in accordance with Good Clinical Practices (GCPs), including International Conference on Harmonization (ICH) Guidelines, and in general conformity with the most recent version of the Declaration of Helsinki. -

The Endocytic Membrane Trafficking Pathway Plays a Major Role
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by University of Liverpool Repository RESEARCH ARTICLE The Endocytic Membrane Trafficking Pathway Plays a Major Role in the Risk of Parkinson’s Disease Sara Bandres-Ciga, PhD,1,2 Sara Saez-Atienzar, PhD,3 Luis Bonet-Ponce, PhD,4 Kimberley Billingsley, MSc,1,5,6 Dan Vitale, MSc,7 Cornelis Blauwendraat, PhD,1 Jesse Raphael Gibbs, PhD,7 Lasse Pihlstrøm, MD, PhD,8 Ziv Gan-Or, MD, PhD,9,10 The International Parkinson’s Disease Genomics Consortium (IPDGC), Mark R. Cookson, PhD,4 Mike A. Nalls, PhD,1,11 and Andrew B. Singleton, PhD1* 1Molecular Genetics Section, Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, Maryland, USA 2Instituto de Investigación Biosanitaria de Granada (ibs.GRANADA), Granada, Spain 3Transgenics Section, Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, Maryland, USA 4Cell Biology and Gene Expression Section, Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, Maryland, USA 5Department of Molecular and Clinical Pharmacology, Institute of Translational Medicine, University of Liverpool, Liverpool, United Kingdom 6Department of Pathophysiology, University of Tartu, Tartu, Estonia 7Computational Biology Group, Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, Maryland, USA 8Department of Neurology, Oslo University Hospital, Oslo, Norway 9Department of Neurology and Neurosurgery, Department of Human Genetics, McGill University, Montréal, Quebec, Canada 10Department of Neurology and Neurosurgery, Montreal Neurological Institute, McGill University, Montréal, Quebec, Canada 11Data Tecnica International, Glen Echo, Maryland, USA ABSTRACT studies, summary-data based Mendelian randomization Background: PD is a complex polygenic disorder. -

SPRAVATO® Esketamine Hydrochloride DATA SHEET
SPRAVATO® esketamine hydrochloride DATA SHEET 1. PRODUCT NAME SPRAVATO® esketamine hydrochloride 32.3mg (equivalent to 28mg esketamine) nasal spray 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each single use nasal spray device delivers two sprays, one spray into each nostril. Total volume of drug product per device to be delivered is 0.2 mL containing a total of 32.3 mg of esketamine hydrochloride (equivalent to 28 mg of esketamine). For the full list of excipients, see Section 6.1 List of Excipients. 3. PHARMACEUTICAL FORM Nasal spray, solution. Clear, colourless, aqueous solution. 4. CLINICAL PARTICULARS 4.1 Therapeutic Indications SPRAVATO in conjunction with an oral antidepressant, is indicated for: - treatment resistant depression (Major Depressive Disorder in adults who have not responded adequately to at least two different antidepressants of adequate dose and duration to treat the current depressive episode). - rapid reduction of depressive symptoms in patients with Major Depressive Disorder who have acute suicidal ideation or behaviour. SPRAVATO is not indicated to prevent suicide or in reducing suicidal ideation or behaviour (see section 4.4. Special Warnings And Precautions For Use) 4.2 Dose and method of administration In patients with TRD, SPRAVATO should be administered in conjunction with a newly initiated oral antidepressant (AD) therapy. In patients with MDSI, SPRAVATO should be administered in conjunction with standard of care therapy. During the Phase III MDSI clinical program, standard of care oral AD treatment (either AD monotherapy or AD plus augmentation therapy) was initiated or optimised. Important Considerations Prior to Initiating and During Therapy SPRAVATO must be administered under the direct supervision of a healthcare provider. -

Sciatica and Chronic Pain
Sciatica and Chronic Pain Past, Present and Future Robert W. Baloh 123 Sciatica and Chronic Pain Robert W. Baloh Sciatica and Chronic Pain Past, Present and Future Robert W. Baloh, MD Department of Neurology University of California, Los Angeles Los Angeles, CA, USA ISBN 978-3-319-93903-2 ISBN 978-3-319-93904-9 (eBook) https://doi.org/10.1007/978-3-319-93904-9 Library of Congress Control Number: 2018952076 © Springer International Publishing AG, part of Springer Nature 2019 This work is subject to copyright. All rights are reserved by the Publisher, whether the whole or part of the material is concerned, specifically the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfilms or in any other physical way, and transmission or information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed. The use of general descriptive names, registered names, trademarks, service marks, etc. in this publication does not imply, even in the absence of a specific statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use. The publisher, the authors, and the editors are safe to assume that the advice and information in this book are believed to be true and accurate at the date of publication. Neither the publisher nor the authors or the editors give a warranty, express or implied, with respect to the material contained herein or for any errors or omissions that may have been made. The publisher remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. -

Supplementary Table 1: Adhesion Genes Data Set
Supplementary Table 1: Adhesion genes data set PROBE Entrez Gene ID Celera Gene ID Gene_Symbol Gene_Name 160832 1 hCG201364.3 A1BG alpha-1-B glycoprotein 223658 1 hCG201364.3 A1BG alpha-1-B glycoprotein 212988 102 hCG40040.3 ADAM10 ADAM metallopeptidase domain 10 133411 4185 hCG28232.2 ADAM11 ADAM metallopeptidase domain 11 110695 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 195222 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 165344 8751 hCG20021.3 ADAM15 ADAM metallopeptidase domain 15 (metargidin) 189065 6868 null ADAM17 ADAM metallopeptidase domain 17 (tumor necrosis factor, alpha, converting enzyme) 108119 8728 hCG15398.4 ADAM19 ADAM metallopeptidase domain 19 (meltrin beta) 117763 8748 hCG20675.3 ADAM20 ADAM metallopeptidase domain 20 126448 8747 hCG1785634.2 ADAM21 ADAM metallopeptidase domain 21 208981 8747 hCG1785634.2|hCG2042897 ADAM21 ADAM metallopeptidase domain 21 180903 53616 hCG17212.4 ADAM22 ADAM metallopeptidase domain 22 177272 8745 hCG1811623.1 ADAM23 ADAM metallopeptidase domain 23 102384 10863 hCG1818505.1 ADAM28 ADAM metallopeptidase domain 28 119968 11086 hCG1786734.2 ADAM29 ADAM metallopeptidase domain 29 205542 11085 hCG1997196.1 ADAM30 ADAM metallopeptidase domain 30 148417 80332 hCG39255.4 ADAM33 ADAM metallopeptidase domain 33 140492 8756 hCG1789002.2 ADAM7 ADAM metallopeptidase domain 7 122603 101 hCG1816947.1 ADAM8 ADAM metallopeptidase domain 8 183965 8754 hCG1996391 ADAM9 ADAM metallopeptidase domain 9 (meltrin gamma) 129974 27299 hCG15447.3 ADAMDEC1 ADAM-like, -

A Human Stem Cell-Derived Test System for Agents Modifying Neuronal N
Archives of Toxicology (2021) 95:1703–1722 https://doi.org/10.1007/s00204-021-03024-0 IN VITRO SYSTEMS A human stem cell‑derived test system for agents modifying neuronal 2+ N‑methyl‑D‑aspartate‑type glutamate receptor Ca ‑signalling Stefanie Klima1,2 · Markus Brüll1 · Anna‑Sophie Spreng1,3 · Ilinca Suciu1,3 · Tjalda Falt1 · Jens C. Schwamborn4 · Tanja Waldmann1 · Christiaan Karreman1 · Marcel Leist1,5 Received: 28 October 2020 / Accepted: 4 March 2021 / Published online: 13 March 2021 © The Author(s) 2021 Abstract Methods to assess neuronal receptor functions are needed in toxicology and for drug development. Human-based test systems that allow studies on glutamate signalling are still scarce. To address this issue, we developed and characterized pluripotent stem cell (PSC)-based neural cultures capable of forming a functional network. Starting from a stably proliferating neu- roepithelial stem cell (NESC) population, we generate “mixed cortical cultures” (MCC) within 24 days. Characterization by immunocytochemistry, gene expression profling and functional tests (multi-electrode arrays) showed that MCC contain various functional neurotransmitter receptors, and in particular, the N-methyl-D-aspartate subtype of ionotropic glutamate receptors (NMDA-R). As this important receptor is found neither on conventional neural cell lines nor on most stem cell- derived neurons, we focused here on the characterization of rapid glutamate-triggered Ca2+ signalling. Changes of the intra- 2+ cellular free calcium ion concentration ([Ca ]i) were measured by fuorescent imaging as the main endpoint, and a method to evaluate and quantify signals in hundreds of cells at the same time was developed. We observed responses to glutamate in the low µM range. -

Análise Integrativa De Perfis Transcricionais De Pacientes Com
UNIVERSIDADE DE SÃO PAULO FACULDADE DE MEDICINA DE RIBEIRÃO PRETO PROGRAMA DE PÓS-GRADUAÇÃO EM GENÉTICA ADRIANE FEIJÓ EVANGELISTA Análise integrativa de perfis transcricionais de pacientes com diabetes mellitus tipo 1, tipo 2 e gestacional, comparando-os com manifestações demográficas, clínicas, laboratoriais, fisiopatológicas e terapêuticas Ribeirão Preto – 2012 ADRIANE FEIJÓ EVANGELISTA Análise integrativa de perfis transcricionais de pacientes com diabetes mellitus tipo 1, tipo 2 e gestacional, comparando-os com manifestações demográficas, clínicas, laboratoriais, fisiopatológicas e terapêuticas Tese apresentada à Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo para obtenção do título de Doutor em Ciências. Área de Concentração: Genética Orientador: Prof. Dr. Eduardo Antonio Donadi Co-orientador: Prof. Dr. Geraldo A. S. Passos Ribeirão Preto – 2012 AUTORIZO A REPRODUÇÃO E DIVULGAÇÃO TOTAL OU PARCIAL DESTE TRABALHO, POR QUALQUER MEIO CONVENCIONAL OU ELETRÔNICO, PARA FINS DE ESTUDO E PESQUISA, DESDE QUE CITADA A FONTE. FICHA CATALOGRÁFICA Evangelista, Adriane Feijó Análise integrativa de perfis transcricionais de pacientes com diabetes mellitus tipo 1, tipo 2 e gestacional, comparando-os com manifestações demográficas, clínicas, laboratoriais, fisiopatológicas e terapêuticas. Ribeirão Preto, 2012 192p. Tese de Doutorado apresentada à Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo. Área de Concentração: Genética. Orientador: Donadi, Eduardo Antonio Co-orientador: Passos, Geraldo A. 1. Expressão gênica – microarrays 2. Análise bioinformática por module maps 3. Diabetes mellitus tipo 1 4. Diabetes mellitus tipo 2 5. Diabetes mellitus gestacional FOLHA DE APROVAÇÃO ADRIANE FEIJÓ EVANGELISTA Análise integrativa de perfis transcricionais de pacientes com diabetes mellitus tipo 1, tipo 2 e gestacional, comparando-os com manifestações demográficas, clínicas, laboratoriais, fisiopatológicas e terapêuticas. -

Poster Session III, December 6, 2017
Neuropsychopharmacology (2017) 42, S476–S652 © 2017 American College of Neuropsychopharmacology. All rights reserved 0893-133X/17 www.neuropsychopharmacology.org Poster Session III a high-resolution research tomograph (HRRT), and struc- Palm Springs, California, December 3–7, 2017 tural (T1) MRI using a 3-Tesla scanner. PET data analyses were carried out using the validated 2-tissue compartment Sponsorship Statement: Publication of this supplement is model to determine the total volume of distribution (VT) of sponsored by the ACNP. [18F]FEPPA. Individual contributor disclosures may be found within the Results: Results show significant inverse associations (con- abstracts. Part 1: All Financial Involvement with a pharma- trolling for rs6971 genotype) between neuroinflammation ceutical or biotechnology company, a company providing (VTs) and cortical thickness in the right medial prefrontal clinical assessment, scientific, or medical products or compa- cortex [r = -.562, p = .029] and the left dorsolateral nies doing business with or proposing to do business with prefrontal cortex [r = -.629, p = .012](p values are not ACNP over past 2 years (Calendar Years 2014–Present); Part 2: corrected for multiple comparisons). No significant associa- Income Sources & Equity of $10,000 per year or greater tions were found with surface area. (Calendar Years 2014 - Present): List those financial relation- Conclusions: These results, while preliminary, suggest links ships which are listed in part one and have a value greater than between the microglial activation/neuroinflammation and $10,000 per year, OR financial holdings that are listed in part cortical thickness in the dorsolateral prefrontal cortex and one and have a value of $10,000 or greater as of the date of medial prefrontal cortex in AD patients.