Sciatica and Chronic Pain
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
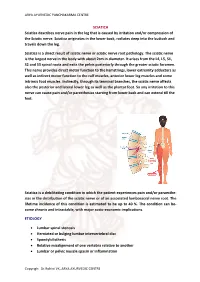
SCIATICA Sciatica Describes Nerve Pain in the Leg That Is Caused by Irritation And/Or Compression of the Sciatic Nerve
ARYA AYURVEDIC PANCHAKARMA CENTRE SCIATICA Sciatica describes nerve pain in the leg that is caused by irritation and/or compression of the Sciatic nerve. Sciatica originates in the lower back, radiates deep into the buttock and travels down the leg. Sciatica is a direct result of sciatic nerve or sciatic nerve root pathology. The sciatic nerve is the largest nerve in the body with about 2cm in diameter. It arises from the L4, L5, S1, S2 and S3 spinal roots and exits the pelvis posteriorly through the greater sciatic foramen. This nerve provides direct motor function to the hamstrings, lower extremity adductors as well as indirect motor function to the calf muscles, anterior lower leg muscles and some intrinsic foot muscles. Indirectly, through its terminal branches, the sciatic nerve affects also the posterior and lateral lower leg as well as the plantar foot. So any irritation to this nerve can cause pain and/or paresthesias starting from lower back and can extend till the feet. Sciatica is a debilitating condition in which the patient experiences pain and/or paraesthe- sias in the distribution of the sciatic nerve or of an associated lumbosacral nerve root. The lifetime incidence of this condition is estimated to be up to 40 %. The condition can be- come chronic and intractable, with major socio-economic implications. ETIOLOGY • Lumbar spinal stenosis • Herniated or bulging lumbar intervertebral disc • Spondylolisthesis • Relative misalignment of one vertebra relative to another • Lumbar or pelvic muscle spasm or inflammation Copyrigh: Dr.Rohini VK, ARYA AYURVEDIC CENTRE ARYA AYURVEDIC PANCHAKARMA CENTRE • Spinal or Paraspinal masses included malignancy, epidural haematoma or epidural abscess • Lumbar degenerative disc disease • Sacroiliac joint dysfunction EPIDEMIOLOGY GENDER: There appears to be no gender predominance. -

Radiculopathy Vs. Spinal Stenosis: Evocative Electrodiagnosis Identifies the Main Pain Generator
Functional Electromyography Loren M. Fishman · Allen N. Wilkins Functional Electromyography Provocative Maneuvers in Electrodiagnosis 123 Loren M. Fishman, MD Allen N. Wilkins, MD College of Physicians & Surgeons Manhattan Physical Medicine Columbia University and Rehabilitation New York, NY 10028, USA New York, NY 10013, USA [email protected] ISBN 978-1-60761-019-9 e-ISBN 978-1-60761-020-5 DOI 10.1007/978-1-60761-020-5 Springer New York Dordrecht Heidelberg London Library of Congress Control Number: 2010935087 © Springer Science+Business Media, LLC 2011 All rights reserved. This work may not be translated or copied in whole or in part without the written permission of the publisher (Springer Science+Business Media, LLC, 233 Spring Street, New York, NY 10013, USA), except for brief excerpts in connection with reviews or scholarly analysis. Use in connection with any form of information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed is forbidden. The use in this publication of trade names, trademarks, service marks, and similar terms, even if they are not identified as such, is not to be taken as an expression of opinion as to whether or not they are subject to proprietary rights. While the advice and information in this book are believed to be true and accurate at the date of going to press, neither the authors nor the editors nor the publisher can accept any legal responsibility for any errors or omissions that may be made. The publisher makes no warranty, express or implied, with respect to the material contained herein. -

Domenico Cotugno E Antonio Miglietta: Dal Protomedicato Al Comitato Centrale Di Vaccinazione
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by ESE - Salento University Publishing L'IDOMENEO Idomeneo (2014), n. 17, 153-174 ISSN 2038-0313 DOI 10.1285/i20380313v17p153 http://siba-ese.unisalento.it, © 2014 Università del Salento Domenico Cotugno e Antonio Miglietta: dal Protomedicato al Comitato centrale di vaccinazione Antonio Borrelli 1. I rapporti fra Domenico Cotugno, il più celebre medico e scienziato meridionale tra Sette e Ottocento, e Antonio Miglietta, il principale artefice della pratica vaccinica nel Regno delle Due Sicilie, sono stati solo accennati da qualche studioso e solo per la loro contemporanea partecipazione al Comitato centrale di vaccinazione, sorto in epoca francese1. In realtà i rapporti fra i due furono molto più intensi e riguardarono, in particolare, il loro contributo alla riforma del Protomedicato, una istituzione che agli inizi dell’Ottocento versava in una crisi profonda, dalla quale, al di là degli sforzi dei singoli che ne fecero parte, non si riprese più. Fra Cotugno e Miglietta, entrambi di origine pugliese, vi era una notevole differenza di età. Il primo era nato a Ruvo di Puglia, in una modesta famiglia di agricoltori, il 29 gennaio 17362; il secondo a Carmiano, presso Otranto, in una famiglia appartenente alla piccola nobiltà, l’8 dicembre 17673. Un periodo che fu di grande rilevanza per le sorti del Regno delle Due Sicilie. Divenuto autonomo nel 1734 con Carlo di Borbone, fu governato dal 1759, dopo la partenza del sovrano per la Spagna, dal figlio Ferdinando che, avendo solo otto anni, fu affiancato da un consiglio di reggenza, tra i cui membri figurava Bernardo Tanucci. -

Piriformis Syndrome: the Literal “Pain in My Butt” Chelsea Smith, PTA
Piriformis Syndrome: the literal “pain in my butt” Chelsea Smith, PTA Aside from the monotony of day-to-day pains and annoyances, piriformis syndrome is the literal “pain in my butt” that may not go away with sending the kids to grandmas and often takes the form of sciatica. Many individuals with pain in the buttock that radiates down the leg are experiencing a form of sciatica caused by irritation of the spinal nerves in or near the lumbar spine (1). Other times though, the nerve irritation is not in the spine but further down the leg due to a pesky muscle called the piriformis, hence “piriformis syndrome”. The piriformis muscle is a flat, pyramidal-shaped muscle that originates from the front surface of the sacrum and the joint capsule of the sacroiliac joint (SI joint) and is located deep in the gluteal tissue (2). The piriformis travels through the greater sciatic foramen and attaches to the upper surface of the greater trochanter (or top of the hip bone) while the sciatic nerve runs under (and sometimes through) the piriformis muscle as it exits the pelvis. Due to this close proximity between the piriformis muscle and the sciatic nerve, if there is excessive tension (tightness), spasm, or inflammation of the piriformis muscle this can cause irritation to the sciatic nerve leading to symptoms of sciatica (pain down the leg) (1). Activities like sitting on hard surfaces, crouching down, walking or running for long distances, and climbing stairs can all increase symptoms (2) with the most common symptom being tenderness along the piriformis muscle (deep in the gluteal region) upon palpation. -

Piriformis Syndrome Is Overdiagnosed 11 Robert A
American Association of Neuromuscular & Electrodiagnostic Medicine AANEM CROSSFIRE: CONTROVERSIES IN NEUROMUSCULAR AND ELECTRODIAGNOSTIC MEDICINE Loren M. Fishman, MD, B.Phil Robert A.Werner, MD, MS Scott J. Primack, DO Willam S. Pease, MD Ernest W. Johnson, MD Lawrence R. Robinson, MD 2005 AANEM COURSE F AANEM 52ND Annual Scientific Meeting Monterey, California CROSSFIRE: Controversies in Neuromuscular and Electrodiagnostic Medicine Loren M. Fishman, MD, B.Phil Robert A.Werner, MD, MS Scott J. Primack, DO Willam S. Pease, MD Ernest W. Johnson, MD Lawrence R. Robinson, MD 2005 COURSE F AANEM 52nd Annual Scientific Meeting Monterey, California AANEM Copyright © September 2005 American Association of Neuromuscular & Electrodiagnostic Medicine 421 First Avenue SW, Suite 300 East Rochester, MN 55902 PRINTED BY JOHNSON PRINTING COMPANY, INC. ii CROSSFIRE: Controversies in Neuromuscular and Electrodiagnostic Medicine Faculty Loren M. Fishman, MD, B.Phil Scott J. Primack, DO Assistant Clinical Professor Co-director Department of Physical Medicine and Rehabilitation Colorado Rehabilitation and Occupational Medicine Columbia College of Physicians and Surgeons Denver, Colorado New York City, New York Dr. Primack completed his residency at the Rehabilitation Institute of Dr. Fishman is a specialist in low back pain and sciatica, electrodiagnosis, Chicago in 1992. He then spent 6 months with Dr. Larry Mack at the functional assessment, and cognitive rehabilitation. Over the last 20 years, University of Washington. Dr. Mack, in conjunction with the Shoulder he has lectured frequently and contributed over 55 publications. His most and Elbow Service at the University of Washington, performed some of the recent work, Relief is in the Stretch: End Back Pain Through Yoga, and the original research utilizing musculoskeletal ultrasound in order to diagnose earlier book, Back Talk, both written with Carol Ardman, were published shoulder pathology. -

Torticollis Is a Clinical Diagnosis Based on Head Tilt in Association with a Rotatory Deviation of the Cranium
24. Define type of torticollis? Torticollis is a clinical diagnosis based on head tilt in association with a rotatory deviation of the cranium. Congenital muscular torticollis is the most common type of torticollis. It presents in the newborn period. Its cause is unknown, but it has been hypothesized to arise from compression of the soft tissues of the neck during delivery, resulting in a compartment syndrome. Radiographs of the cervical spine should be obtained to rule out congenital vertebral anomalies. Clinical examination reveals spasm of the sternocleidomastoid muscle on the same side as the tilt causing the typical posture of head tilt toward the tightened muscle and chin rotation to the opposite side. Initial treatment is stretching and is successful in up to 90% of patients during the first year of life. Surgery is considered for persistent deformity after 1 year of age. Common problems noted in patients with congenital muscular torticollis include congenital hip dysplasia and plagiocephaly (facial asymmetry). Source: Spine Secret Plus, 2nd ed. page 256. 25. What clinical problems result from basilar impression? Patients present with a short neck, painful cervical motion, and asymmetric of the skull and face. Additional clinical problems include nuchal pain, vertigo, long tract signs with associated cerebellar ataxia, and lower cranial nerve involvement resulting in dysarthria and dysphagia Source: Spine Secret Plus, 2nd ed. page 258 26. What is Steel’s Rule of Third? Steel noted that the area of the spinal canal at the C1 leve in a normal person could be divided into equal thirds with one third occupied by the odontoid process, one third by the spinal cord, and one third as empty space. -

The Teaching of Anatomy Throughout the Centuries: from Herophilus To
Medicina Historica 2019; Vol. 3, N. 2: 69-77 © Mattioli 1885 Original article: history of medicine The teaching of anatomy throughout the centuries: from Herophilus to plastination and beyond Veronica Papa1, 2, Elena Varotto2, 3, Mauro Vaccarezza4, Roberta Ballestriero5, 6, Domenico Tafuri1, Francesco M. Galassi2, 7 1 Department of Motor Sciences and Wellness, University of Naples “Parthenope”, Napoli, Italy; 2 FAPAB Research Center, Avola (SR), Italy; 3 Department of Humanities (DISUM), University of Catania, Catania, Italy; 4 School of Pharmacy and Biomedical Sciences, Faculty of Health Sciences, Curtin University, Bentley, Perth, WA, Australia; 5 University of the Arts, Central Saint Martins, London, UK; 6 The Gordon Museum of Pathology, Kings College London, London, UK;7 Archaeology, College of Hu- manities, Arts and Social Sciences, Flinders University, Adelaide, Australia Abstract. Cultural changes, scientific progress, and new trends in medical education have modified the role of dissection in the teaching of anatomy in today’s medical schools. Dissection is indispensable for a correct and complete knowledge of human anatomy, which can ensure safe as well as efficient clinical practice and the hu- man dissection lab could possibly be the ideal place to cultivate humanistic qualities among future physicians. In this manuscript, we discuss the role of dissection itself, the value of which has been under debate for the last 30 years; furthermore, we attempt to focus on the way in which anatomy knowledge was delivered throughout the centuries, from the ancient times, through the Middles Ages to the present. Finally, we document the rise of plastination as a new trend in anatomy education both in medical and non-medical practice. -

A Human Stem Cell-Derived Test System for Agents Modifying Neuronal N
Archives of Toxicology (2021) 95:1703–1722 https://doi.org/10.1007/s00204-021-03024-0 IN VITRO SYSTEMS A human stem cell‑derived test system for agents modifying neuronal 2+ N‑methyl‑D‑aspartate‑type glutamate receptor Ca ‑signalling Stefanie Klima1,2 · Markus Brüll1 · Anna‑Sophie Spreng1,3 · Ilinca Suciu1,3 · Tjalda Falt1 · Jens C. Schwamborn4 · Tanja Waldmann1 · Christiaan Karreman1 · Marcel Leist1,5 Received: 28 October 2020 / Accepted: 4 March 2021 / Published online: 13 March 2021 © The Author(s) 2021 Abstract Methods to assess neuronal receptor functions are needed in toxicology and for drug development. Human-based test systems that allow studies on glutamate signalling are still scarce. To address this issue, we developed and characterized pluripotent stem cell (PSC)-based neural cultures capable of forming a functional network. Starting from a stably proliferating neu- roepithelial stem cell (NESC) population, we generate “mixed cortical cultures” (MCC) within 24 days. Characterization by immunocytochemistry, gene expression profling and functional tests (multi-electrode arrays) showed that MCC contain various functional neurotransmitter receptors, and in particular, the N-methyl-D-aspartate subtype of ionotropic glutamate receptors (NMDA-R). As this important receptor is found neither on conventional neural cell lines nor on most stem cell- derived neurons, we focused here on the characterization of rapid glutamate-triggered Ca2+ signalling. Changes of the intra- 2+ cellular free calcium ion concentration ([Ca ]i) were measured by fuorescent imaging as the main endpoint, and a method to evaluate and quantify signals in hundreds of cells at the same time was developed. We observed responses to glutamate in the low µM range. -

ICD9 & ICD10 Neuromuscular Codes
ICD-9-CM and ICD-10-CM NEUROMUSCULAR DIAGNOSIS CODES ICD-9-CM ICD-10-CM Focal Neuropathy Mononeuropathy G56.00 Carpal tunnel syndrome, unspecified Carpal tunnel syndrome 354.00 G56.00 upper limb Other lesions of median nerve, Other median nerve lesion 354.10 G56.10 unspecified upper limb Lesion of ulnar nerve, unspecified Lesion of ulnar nerve 354.20 G56.20 upper limb Lesion of radial nerve, unspecified Lesion of radial nerve 354.30 G56.30 upper limb Lesion of sciatic nerve, unspecified Sciatic nerve lesion (Piriformis syndrome) 355.00 G57.00 lower limb Meralgia paresthetica, unspecified Meralgia paresthetica 355.10 G57.10 lower limb Lesion of lateral popiteal nerve, Peroneal nerve (lesion of lateral popiteal nerve) 355.30 G57.30 unspecified lower limb Tarsal tunnel syndrome, unspecified Tarsal tunnel syndrome 355.50 G57.50 lower limb Plexus Brachial plexus lesion 353.00 Brachial plexus disorders G54.0 Brachial neuralgia (or radiculitis NOS) 723.40 Radiculopathy, cervical region M54.12 Radiculopathy, cervicothoracic region M54.13 Thoracic outlet syndrome (Thoracic root Thoracic root disorders, not elsewhere 353.00 G54.3 lesions, not elsewhere classified) classified Lumbosacral plexus lesion 353.10 Lumbosacral plexus disorders G54.1 Neuralgic amyotrophy 353.50 Neuralgic amyotrophy G54.5 Root Cervical radiculopathy (Intervertebral disc Cervical disc disorder with myelopathy, 722.71 M50.00 disorder with myelopathy, cervical region) unspecified cervical region Lumbosacral root lesions (Degeneration of Other intervertebral disc degeneration, -

Diagnosing and Treating Trigeminal Neuralgia in General Dentistry
general practice feature Chasing Pain Diagnosing and Treating Trigeminal Neuralgia in General Dentistry by Steven Olmos, DDS, DABCP, DABCDSM, DABDSM, DAAPM, FAAOP, FAACP, FICCMO, FADI, FIAO As dentists, we know quite a bit about tooth and gum pain, but when it comes to chronic facial pain and neuropathic pain, our dental school education leaves us unprepared. The objective of this article is to explain the differences between men and women with chronic orofacial pain and the relationship to proper functional breathing, using a case study as demonstration. 34 JANUARY 2016 // dentaltown.com general practice feature the United States, nearly half research published in Chest 2015 demonstrates that of all adults lived with chronic respiratory-effort-related arousal may be the most pain in 2011. Of 353,000 adults likely cause (nasal obstruction or mouth breath- 11 aged 18 years or older who were ing). Rising C02 (hypercapnia) in a patient with a surveyed by Gallup-Health- sleep-breathing disorder (including mouth breath- ways, 47 percent reported having at least one of ing) specifically stimulates the superficial masseter three types of chronic pain: neck or back pain, muscles to contract.12 knee or leg pain, or recurring pain.2 Identifying the structural area of obstruction A study published in The Journal of the Amer- (Four Points of Obstruction; Fig. 1) of the air- ican Dental Association October 2015 stated: way will insure the most effective treatment for a “One in six patients visiting a general dentist had sleep-breathing disorder and effectively reduce the experienced orofacial pain during the last year. -

The Hip's Influence on Low Back Pain
Journal of Sport Rehabilitation, 2009, 18, 24-32 © 2009 Human Kinetics, Inc. The Hip’s Influence on Low Back Pain: A Distal Link to a Proximal Problem Michael P. Reiman, P. Cody Weisbach, and Paul E. Glynn Low back pain (LBP) is a multifactorial dysfunction, with one of the potential con- tributing factors being the hip joint. Currently, research investigating the examination and conservative treatment of LBP has focused primarily on the lumbar spine. The objective of this clinical commentary is to discuss the potential link between hip impairments and LBP using current best evidence and the concept of regional inter- dependence as tools to guide decision making and offer ideas for future research. Keywords: strength, rehabilitation In day-to-day clinical practice it is often difficult to identify the source of symptoms in patients with low back pain (LBP).1 Abenhaim et al2 noted that a small percentage of individuals with LBP have an identifiable pathoanatomical source. Further clouding the picture are multiple studies indicating the potential inability of diagnostic imaging to identify the pain source, influence prognosis, or affect outcomes.3–6 Research has demonstrated the effectiveness of subgrouping patients into a classification system based on signs and symptoms indicating their likelihood to respond to specific treatments. This classification approach has pro- duced improved outcomes and high levels of reliability as compared with clinical- practice guidelines.7–9 For treating clinicians, these findings help guide decision making and improve results; however, not all patients will fit into a treatment- based subgroup. The treating therapist must then rely on an impairment-based approach, identifying potential local or remote contributors to the patient’s area of primary concern. -

Occipital Neuralgia: a Literature Review of Current Treatments from Traditional Medicine to CAM Treatments
Occipital Neuralgia: A Literature Review of Current Treatments from Traditional Medicine to CAM Treatments By Nikole Benavides Faculty Advisor: Dr. Patrick Montgomery Graduation: April 2011 1 Abstract Objective. This article provides an overview of the current and upcoming treatments for people who suffer from the signs and symptoms of greater occipital neuralgia. Types of treatments will be analyzed and discussed, varying from traditional Western medicine to treatments from complementary and alternative health care. Methods. A PubMed search was performed using the key words listed in this abstract. Results. Twenty-nine references were used in this literature review. The current literature reveals abundant peer reviewed research on medications used to treat this malady, but relatively little on the CAM approach. Conclusion. Occipital Neuralgia has become one of the more complicated headaches to diagnose. The symptoms often mimic those of other headaches and can occur post-trauma or due to other contributing factors. There are a variety of treatments that involve surgery or blocking of the greater occipital nerve. As people continue to seek more natural treatments, the need for alternative treatments is on the rise. Key Words. Occipital Neuralgia; Headache; Alternative Treatments; Acupuncture; Chiropractic; Nutrition 2 Introduction Occipital neuralgia is a type of headache that describes the irritation of the greater occipital nerve and the signs and symptoms associated with it. It is a difficult headache to diagnose due to the variety of signs and symptoms it presents with. It can be due to a post-traumatic event, degenerative changes, congenital anomalies, or other factors (10). The patterns of occipital neuralgia mimic those of other headaches.