Surgery for Lumbar Radiculopathy/ Sciatica Final Evidence Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Peroneal Neuropathy Misdiagnosed As L5 Radiculopathy: a Case Report Michael D Reife1,2* and Christopher M Coulis3,4,5
Reife and Coulis Chiropractic & Manual Therapies 2013, 21:12 http://www.chiromt.com/content/21/1/12 CHIROPRACTIC & MANUAL THERAPIES CASE REPORT Open Access Peroneal neuropathy misdiagnosed as L5 radiculopathy: a case report Michael D Reife1,2* and Christopher M Coulis3,4,5 Abstract Objective: The purpose of this case report is to describe a patient who presented with a case of peroneal neuropathy that was originally diagnosed and treated as a L5 radiculopathy. Clinical features: A 53-year old female registered nurse presented to a private chiropractic practice with complaints of left lateral leg pain. Three months earlier she underwent elective left L5 decompression surgery without relief of symptoms. Intervention and outcome: Lumbar spine MRI seven months prior to lumbar decompression surgery revealed left neural foraminal stenosis at L5-S1. The patient symptoms resolved after she stopped crossing her legs. Conclusion: This report discusses a case of undiagnosed peroneal neuropathy that underwent lumbar decompression surgery for a L5 radiculopathy. This case study demonstrates the importance of a thorough clinical examination and decision making that ensures proper patient diagnosis and management. Keywords: Peroneal neuropathy, Lumbar radiculopathy, Chiropractic Background Case presentation The most common entrapment neuropathy in the lo- The patient is a 53-year-old registered nurse who was wer extremity is common peroneal mononeuropathy, ac- referred to the author’s office in August 2003 by her pri- counting for approximately 15% of all mononeuropathies mary care physician with a chief complaint of left leg in adults [1]. Most injuries occur at the fibular head and pain. Her symptoms began in October 2002 after she fell can be the result of many factors including chronic low off an ambulance landing on her left hip. -

Vertebral Column and Thorax
Introduction to Human Osteology Chapter 4: Vertebral Column and Thorax Roberta Hall Kenneth Beals Holm Neumann Georg Neumann Gwyn Madden Revised in 1978, 1984, and 2008 The Vertebral Column and Thorax Sternum Manubrium – bone that is trapezoidal in shape, makes up the superior aspect of the sternum. Jugular notch – concave notches on either side of the superior aspect of the manubrium, for articulation with the clavicles. Corpus or body – flat, rectangular bone making up the major portion of the sternum. The lateral aspects contain the notches for the true ribs, called the costal notches. Xiphoid process – variably shaped bone found at the inferior aspect of the corpus. Process may fuse late in life to the corpus. Clavicle Sternal end – rounded end, articulates with manubrium. Acromial end – flat end, articulates with scapula. Conoid tuberosity – muscle attachment located on the inferior aspect of the shaft, pointing posteriorly. Ribs Scapulae Head Ventral surface Neck Dorsal surface Tubercle Spine Shaft Coracoid process Costal groove Acromion Glenoid fossa Axillary margin Medial angle Vertebral margin Manubrium. Left anterior aspect, right posterior aspect. Sternum and Xyphoid Process. Left anterior aspect, right posterior aspect. Clavicle. Left side. Top superior and bottom inferior. First Rib. Left superior and right inferior. Second Rib. Left inferior and right superior. Typical Rib. Left inferior and right superior. Eleventh Rib. Left posterior view and left superior view. Twelfth Rib. Top shows anterior view and bottom shows posterior view. Scapula. Left side. Top anterior and bottom posterior. Scapula. Top lateral and bottom superior. Clavicle Sternum Scapula Ribs Vertebrae Body - Development of the vertebrae can be used in aging of individuals. -
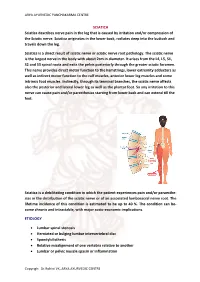
SCIATICA Sciatica Describes Nerve Pain in the Leg That Is Caused by Irritation And/Or Compression of the Sciatic Nerve
ARYA AYURVEDIC PANCHAKARMA CENTRE SCIATICA Sciatica describes nerve pain in the leg that is caused by irritation and/or compression of the Sciatic nerve. Sciatica originates in the lower back, radiates deep into the buttock and travels down the leg. Sciatica is a direct result of sciatic nerve or sciatic nerve root pathology. The sciatic nerve is the largest nerve in the body with about 2cm in diameter. It arises from the L4, L5, S1, S2 and S3 spinal roots and exits the pelvis posteriorly through the greater sciatic foramen. This nerve provides direct motor function to the hamstrings, lower extremity adductors as well as indirect motor function to the calf muscles, anterior lower leg muscles and some intrinsic foot muscles. Indirectly, through its terminal branches, the sciatic nerve affects also the posterior and lateral lower leg as well as the plantar foot. So any irritation to this nerve can cause pain and/or paresthesias starting from lower back and can extend till the feet. Sciatica is a debilitating condition in which the patient experiences pain and/or paraesthe- sias in the distribution of the sciatic nerve or of an associated lumbosacral nerve root. The lifetime incidence of this condition is estimated to be up to 40 %. The condition can be- come chronic and intractable, with major socio-economic implications. ETIOLOGY • Lumbar spinal stenosis • Herniated or bulging lumbar intervertebral disc • Spondylolisthesis • Relative misalignment of one vertebra relative to another • Lumbar or pelvic muscle spasm or inflammation Copyrigh: Dr.Rohini VK, ARYA AYURVEDIC CENTRE ARYA AYURVEDIC PANCHAKARMA CENTRE • Spinal or Paraspinal masses included malignancy, epidural haematoma or epidural abscess • Lumbar degenerative disc disease • Sacroiliac joint dysfunction EPIDEMIOLOGY GENDER: There appears to be no gender predominance. -

Radiculopathy Vs. Spinal Stenosis: Evocative Electrodiagnosis Identifies the Main Pain Generator
Functional Electromyography Loren M. Fishman · Allen N. Wilkins Functional Electromyography Provocative Maneuvers in Electrodiagnosis 123 Loren M. Fishman, MD Allen N. Wilkins, MD College of Physicians & Surgeons Manhattan Physical Medicine Columbia University and Rehabilitation New York, NY 10028, USA New York, NY 10013, USA [email protected] ISBN 978-1-60761-019-9 e-ISBN 978-1-60761-020-5 DOI 10.1007/978-1-60761-020-5 Springer New York Dordrecht Heidelberg London Library of Congress Control Number: 2010935087 © Springer Science+Business Media, LLC 2011 All rights reserved. This work may not be translated or copied in whole or in part without the written permission of the publisher (Springer Science+Business Media, LLC, 233 Spring Street, New York, NY 10013, USA), except for brief excerpts in connection with reviews or scholarly analysis. Use in connection with any form of information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed is forbidden. The use in this publication of trade names, trademarks, service marks, and similar terms, even if they are not identified as such, is not to be taken as an expression of opinion as to whether or not they are subject to proprietary rights. While the advice and information in this book are believed to be true and accurate at the date of going to press, neither the authors nor the editors nor the publisher can accept any legal responsibility for any errors or omissions that may be made. The publisher makes no warranty, express or implied, with respect to the material contained herein. -

Brachial-Plexopathy.Pdf
Brachial Plexopathy, an overview Learning Objectives: The brachial plexus is the network of nerves that originate from cervical and upper thoracic nerve roots and eventually terminate as the named nerves that innervate the muscles and skin of the arm. Brachial plexopathies are not common in most practices, but a detailed knowledge of this plexus is important for distinguishing between brachial plexopathies, radiculopathies and mononeuropathies. It is impossible to write a paper on brachial plexopathies without addressing cervical radiculopathies and root avulsions as well. In this paper will review brachial plexus anatomy, clinical features of brachial plexopathies, differential diagnosis, specific nerve conduction techniques, appropriate protocols and case studies. The reader will gain insight to this uncommon nerve problem as well as the importance of the nerve conduction studies used to confirm the diagnosis of plexopathies. Anatomy of the Brachial Plexus: To assess the brachial plexus by localizing the lesion at the correct level, as well as the severity of the injury requires knowledge of the anatomy. An injury involves any condition that impairs the function of the brachial plexus. The plexus is derived of five roots, three trunks, two divisions, three cords, and five branches/nerves. Spinal roots join to form the spinal nerve. There are dorsal and ventral roots that emerge and carry motor and sensory fibers. Motor (efferent) carries messages from the brain and spinal cord to the peripheral nerves. This Dorsal Root Sensory (afferent) carries messages from the peripheral to the Ganglion is why spinal cord or both. A small ganglion containing cell bodies of sensory NCS’s sensory fibers lies on each posterior root. -

Piriformis Syndrome: the Literal “Pain in My Butt” Chelsea Smith, PTA
Piriformis Syndrome: the literal “pain in my butt” Chelsea Smith, PTA Aside from the monotony of day-to-day pains and annoyances, piriformis syndrome is the literal “pain in my butt” that may not go away with sending the kids to grandmas and often takes the form of sciatica. Many individuals with pain in the buttock that radiates down the leg are experiencing a form of sciatica caused by irritation of the spinal nerves in or near the lumbar spine (1). Other times though, the nerve irritation is not in the spine but further down the leg due to a pesky muscle called the piriformis, hence “piriformis syndrome”. The piriformis muscle is a flat, pyramidal-shaped muscle that originates from the front surface of the sacrum and the joint capsule of the sacroiliac joint (SI joint) and is located deep in the gluteal tissue (2). The piriformis travels through the greater sciatic foramen and attaches to the upper surface of the greater trochanter (or top of the hip bone) while the sciatic nerve runs under (and sometimes through) the piriformis muscle as it exits the pelvis. Due to this close proximity between the piriformis muscle and the sciatic nerve, if there is excessive tension (tightness), spasm, or inflammation of the piriformis muscle this can cause irritation to the sciatic nerve leading to symptoms of sciatica (pain down the leg) (1). Activities like sitting on hard surfaces, crouching down, walking or running for long distances, and climbing stairs can all increase symptoms (2) with the most common symptom being tenderness along the piriformis muscle (deep in the gluteal region) upon palpation. -

Piriformis Syndrome Is Overdiagnosed 11 Robert A
American Association of Neuromuscular & Electrodiagnostic Medicine AANEM CROSSFIRE: CONTROVERSIES IN NEUROMUSCULAR AND ELECTRODIAGNOSTIC MEDICINE Loren M. Fishman, MD, B.Phil Robert A.Werner, MD, MS Scott J. Primack, DO Willam S. Pease, MD Ernest W. Johnson, MD Lawrence R. Robinson, MD 2005 AANEM COURSE F AANEM 52ND Annual Scientific Meeting Monterey, California CROSSFIRE: Controversies in Neuromuscular and Electrodiagnostic Medicine Loren M. Fishman, MD, B.Phil Robert A.Werner, MD, MS Scott J. Primack, DO Willam S. Pease, MD Ernest W. Johnson, MD Lawrence R. Robinson, MD 2005 COURSE F AANEM 52nd Annual Scientific Meeting Monterey, California AANEM Copyright © September 2005 American Association of Neuromuscular & Electrodiagnostic Medicine 421 First Avenue SW, Suite 300 East Rochester, MN 55902 PRINTED BY JOHNSON PRINTING COMPANY, INC. ii CROSSFIRE: Controversies in Neuromuscular and Electrodiagnostic Medicine Faculty Loren M. Fishman, MD, B.Phil Scott J. Primack, DO Assistant Clinical Professor Co-director Department of Physical Medicine and Rehabilitation Colorado Rehabilitation and Occupational Medicine Columbia College of Physicians and Surgeons Denver, Colorado New York City, New York Dr. Primack completed his residency at the Rehabilitation Institute of Dr. Fishman is a specialist in low back pain and sciatica, electrodiagnosis, Chicago in 1992. He then spent 6 months with Dr. Larry Mack at the functional assessment, and cognitive rehabilitation. Over the last 20 years, University of Washington. Dr. Mack, in conjunction with the Shoulder he has lectured frequently and contributed over 55 publications. His most and Elbow Service at the University of Washington, performed some of the recent work, Relief is in the Stretch: End Back Pain Through Yoga, and the original research utilizing musculoskeletal ultrasound in order to diagnose earlier book, Back Talk, both written with Carol Ardman, were published shoulder pathology. -

5. Vertebral Column
5. Vertebral Column. Human beings belong to a vast group animals, the vertebrates. In simple terms we say that vertebrates are animals with a backbone. This statement barely touches the surface of the issue. Vertebrates are animals with a bony internal skeleton. Besides, all vertebrates have a fundamental common body plan. The central nervous system is closer to the back than it is to the belly, the digestive tube in the middle and the heart is ventral. The body is made of many segments (slices) built to a common plan, but specialised in different regions of the body. A “coelomic cavity” with its own special plan is seen in the trunk region and has a characteristic relationship with the organs in the trunk. This is by no means a complete list of vertebrate characteristics. Moreover, some of these features may be shared by other animal groups in a different manner. Such a study is beyond the scope of this unit. Vertebrates belong to an even wider group of animals, chordates. It may be difficult to imagine that we human beings are in fact related to some the earlier chordates! However, we do share, at least during embryonic development, an important anatomical structure with all chordates. This structure is the notochord. The notochord is the first stiff, internal support that appeared during the evolutionary story. As we have seen in early embryology, it also defines the axis of the body. The vertebral column evolved around the notochord and the neural tube, and we see a reflection of this fact during our embryonic development. -

Neuropathy, Radiculopathy & Myelopathy
Neuropathy, Radiculopathy & Myelopathy Jean D. Francois, MD Neurology & Neurophysiology Purpose and Objectives PURPOSE Avoid Confusing Certain Key Neurologic Concepts OBJECTIVES • Objective 1: Define & Identify certain types of Neuropathies • Objective 2: Define & Identify Radiculopathy & its causes • Objective 3: Define & Identify Myelopathy FINANCIAL NONE DISCLOSURE Basics What is Neuropathy? • The term 'neuropathy' is used to describe a problem with the nerves, usually the 'peripheral nerves' as opposed to the 'central nervous system' (the brain and spinal cord). It refers to Peripheral neuropathy • It covers a wide area and many nerves, but the problem it causes depends on the type of nerves that are affected: • Sensory nerves (the nerves that control sensation>skin) causing cause tingling, pain, numbness, or weakness in the feet and hands • Motor nerves (the nerves that allow power and movement>muscles) causing weakness in the feet and hands • Autonomic nerves (the nerves that control the systems of the body eg gut, bladder>internal organs) causing changes in the heart rate and blood pressure or sweating • It May produce Numbness, tingling,(loss of sensation) along with weakness. It can also cause pain. • It can affect a single nerve (mononeuropathy) or multiple nerves (polyneuropathy) Neuropathy • Symptoms usually start in the longest nerves in the body: Feet & later on the hands (“Stocking-glove” pattern) • Symptoms usually spread slowly and evenly up the legs and arms. Other body parts may also be affected. • Peripheral Neuropathy can affect people of any age. But mostly people over age 55 • CAUSES: Neuropathy has a variety of forms and causes. (an injury systemic illness, an infection, an inherited disorder) some of the causes are still unknown. -

Sciatica and Chronic Pain
Sciatica and Chronic Pain Past, Present and Future Robert W. Baloh 123 Sciatica and Chronic Pain Robert W. Baloh Sciatica and Chronic Pain Past, Present and Future Robert W. Baloh, MD Department of Neurology University of California, Los Angeles Los Angeles, CA, USA ISBN 978-3-319-93903-2 ISBN 978-3-319-93904-9 (eBook) https://doi.org/10.1007/978-3-319-93904-9 Library of Congress Control Number: 2018952076 © Springer International Publishing AG, part of Springer Nature 2019 This work is subject to copyright. All rights are reserved by the Publisher, whether the whole or part of the material is concerned, specifically the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfilms or in any other physical way, and transmission or information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed. The use of general descriptive names, registered names, trademarks, service marks, etc. in this publication does not imply, even in the absence of a specific statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use. The publisher, the authors, and the editors are safe to assume that the advice and information in this book are believed to be true and accurate at the date of publication. Neither the publisher nor the authors or the editors give a warranty, express or implied, with respect to the material contained herein or for any errors or omissions that may have been made. The publisher remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. -

Anatomic Mapping of Lumbar Nerve Roots During a Direct Lateral Transpsoas Approach to the Spine a Cadaveric Study
SPINE Volume 36, Number 11, pp E687–E691 ©2011, Lippincott Williams & Wilkins ANATOMY Anatomic Mapping of Lumbar Nerve Roots During a Direct Lateral Transpsoas Approach to the Spine A Cadaveric Study Kelley Banagan , MD, Daniel Gelb , MD, Kornelis Poelstra , MD, PhD, and Steven Ludwig , MD longitudinal ligament at the level of the disc to the sympathetic chain Study Design. Cadaveric study. averaged 9.25 mm. The nerve roots and genitofemoral nerve were Objective. Identifying anatomic structures at risk for injury during placed at risk in all dissections in which the approach was recreated. direct lateral transpsoas approach to the spine. Damage secondary to K-wire placement occurred in 25% of cases Summary of Background Data. Direct lateral transpsoas at L3–L4 and L4–L5; in one case, L4 nerve root was pierced, and approach is a novel technique that has been described for anterior in another, genitofemoral nerve was pierced. K-wire was posterior lumbar interbody fusion. Potential risks include damage to to the nerve roots in 25% of cases at L3–L4 and in 50% of cases genitofemoral nerve and lumbar plexus, which are not well visualized at L4–L5. The lumbar plexus was placed under tension because of during small retroperitoneal exposure. Previous cadaveric studies did sequential dilator placement. not evaluate the direct lateral transpsoas approach, and considering Conclusion. On the basis of our results, there is no zone of absolute the approach being used in clinical practice, the current study was safety when using the direct lateral transpsoas approach. The potential undertaken in an effort to identify the structures at risk during direct for nerve injury exists when using this approach, and consequently, we lateral transpsoas approach. -

Lumbosacral Plexus Entrapment Syndrome. Part One: a Common Yet Little-Known Cause of Chronic Pelvic and Lower Extremity Pain
3-A Running head: ANAESTHESIA, PAIN & INTENSIVE CARE www.apicareonline.com ORIGINAL ARTICLE Lumbosacral plexus entrapment syndrome. Part one: A common yet little-known cause of chronic pelvic and lower extremity pain Kjetil Larsen, CES, George C. Chang Chien, D O2 ABSTRACT Corrective exercise specialist, Training & Rehabilitation, Oslo Lumbosacral plexus entrapment syndrome (LPES) is a little-known but common cause Norway of chronic lumbopelvic and lower extremity pain. The lumbar plexus, including the 2 Director of pain management, lumbosacral tunks emerge through the fibers of the psoas major, and the proximal Ventura County Medical Center, sciatic nerve beneath the piriformis muscles. Severe weakness of these muscles may Ventura, CA 93003, USA. lead to entrapment plexopathy, resulting in diffuse and non-specific pain patterns Correspondence: Kjetil Larsen, CES, Corrective throughout the lumbopelvic complex and lower extremities (LPLE), easily mimicking Exercise Specialist, Training & other diagnoses and is therefore likely to mislead the interpreting clinician. It is a Rehabilitation, Oslo Norway; pathology very similar to that of thoracic outlet syndrome, but for the lower body. This Kjetil@trainingandrehabilitation. two part manuscript series was written in an attempt to demonstrate the existence, com; pathophysiology, diagnostic protocol as well as interventional strategy for LPES, and Tel.: +47 975 45 192 its efficacy. Received: 23 November 2018, Reviewed & Accepted: 28 Key words: Pelvic girdle; Pain, Pelvic girdle; Lumbosacral plexus entrapment syndrome; February 2019 Piriformis syndrome; Nerve entrapment; Double-crush; Pain, Chronic; Fibromyalgia Citation: Larsen K, Chien GCC. Lumbosacral plexus entrapment syndrome. Part one: A common yet little-known cause of chronic pelvic and lower extremity pain.