Water Quality Monitoring of the Bellinger and Kalang Rivers 2015-16
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
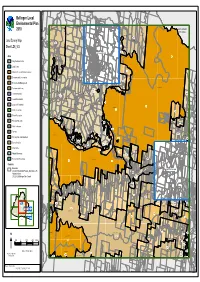
LEP 2010 LZN Template
WILD CATTLE CREEK E1E1E1 DORRIGO RU2RU2 GLENREAGH MULDIVA RU2RU2 OLD BILLINGS RD RAILWAY NATURE COAST TYRINGHAM RESERVE RD RD Bellingen Local E1EE1E11 BORRA CREEK DORRIGO NATIONAL PARK CORAMBA RD E3EE3E33 SLINGSBYS Environmental Plan LITTLE MURRAY RIVER RU2RRU2U2 RD LITTLE PLAIN CREEK BORRA CREEK TYRINGHAM COFFSCOFFS HARBOURHARBOUR 2010 RD CITYCCITYITY COUNCILCOUNCIL BREAKWELLS WILD CATTLE CREEK RD RU2RRU2U2 CORAMBA RD RU2RU2 NEAVES NEAVES RD RD SLINGSBYS RD Land Zoning Map LITTLE MURRAY RIVER OLD COAST RD E3EE3E33 RU2RRU2U2 Sheet LZN_004 E3EE3E33 DEER VALE RD OLD CORAMBA TYRINGHAM RD NTH RD ROCKY CREEK RU1RU1 BARTLETTS RD ReferRefer toto mapmap LZN_004ALZN_004A Zone RU1RRU1U1 BIELSDOWN RIVER E1EE1E11 DEER VALE RD B1 Neighbourhood Centre RU1RU1 DORRIGO NATIONAL PARK B2 Local Centre JOHNSENS WATERFALL WAY OLD CORAMBA WATERFALL WAY RD STH EE3E3E33 E1 National Parks and Nature Reserves E3E3E3 BENNETTS RD E2 Environmental Conservation WATERFALL WAY SHEPHERDS RD RU1RRU1U1 E3 Environmental Management DOME RD EVERINGHAMS ROCKY CREEK RD BIELSDOWN RIVER DORRIGO NATIONAL PARK E4 Environmental Living E3EE3E33 SP1SP1SP1 E3E3E3 SHEPHERDS RD WHISKY CEMETERYCCEMETERYEMETERY DOME RD IN1 General Industrial CREEK RD RU1RRU1U1 RU1RRU1U1 WOODLANDS RD WATERFALL R1 General Residential WAY RU1RRU1U1 PROMISED RU1RU1 E3E3E3 LAND RD WATERFALL WAY R5 Large Lot Residential WHISKY CREEK E1EE1E11 RU1RU1 DORRIGO NATIONAL PARK E1E1E1 E1EE1E11 RE1 Public Recreation BIELSDOWN RIVER E3EE3E33 ROCKY CREEK RD RE2 Private Recreation WHISKY CREEK RD RU1RRU1U1 RU2RU2 E3E3E3 -

Bellinger and Kalang River Estuaries Erosion Study
Bellinger and Kalang River Estuaries Erosion Study Damon Telfer GECO Environmental Tim Cohen IRM Consultants February 2010 Report prep ared for BELLINGEN SHIRE COUNCIL DISCLAIMER Whilst every care has been taken in compiling this erosion study and report, no assurances are given that it is free from error or omission. The authors make no expressed or implied warranty of the accuracy or fitness of the recommendations in the report and disclaim all liability for the consequences of anything done or omitted to be done by any person in reliance upon these recommendations. The use of this report is at the user’s risk and discretion. The authors will not be liable for any loss, damage, costs or injury including consequential, incidental, or financial loss arising out of the use of this report. This report was produced with financial assistance from the NSW Government through the Department of Environment, Climate Change and Water. This document does not necessarily represent the opinions of the NSW Government or the Department of Environment, Climate Change and Water. Damon Telfer GECO Environmental 5 Arcadia Lane, GRASSY HEAD NSW 2441 Email: [email protected] © Damon Telfer, February 2010. This document is copyright and cannot be reproduced in part or whole without the express permission of the author. A permanent, irrevocable royalty-free, non-exclusive license to make these reports, documents and any other materials publicly available and to otherwise communicate, reproduce, adapt and publicise them on a non- profit basis is granted by the authors to Bellingen Shire Council and the Department of Environment, Climate Change and Water. -

Status Review, Disease Risk Analysis and Conservation Action Plan for The
Status Review, Disease Risk Analysis and Conservation Action Plan for the Bellinger River Snapping Turtle (Myuchelys georgesi) December, 2016 1 Workshop participants. Back row (l to r): Ricky Spencer, Bruce Chessman, Kristen Petrov, Caroline Lees, Gerald Kuchling, Jane Hall, Gerry McGilvray, Shane Ruming, Karrie Rose, Larry Vogelnest, Arthur Georges; Front row (l to r) Michael McFadden, Adam Skidmore, Sam Gilchrist, Bruno Ferronato, Richard Jakob-Hoff © Copyright 2017 CBSG IUCN encourages meetings, workshops and other fora for the consideration and analysis of issues related to conservation, and believes that reports of these meetings are most useful when broadly disseminated. The opinions and views expressed by the authors may not necessarily reflect the formal policies of IUCN, its Commissions, its Secretariat or its members. The designation of geographical entities in this book, and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of IUCN concerning the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. Jakob-Hoff, R. Lees C. M., McGilvray G, Ruming S, Chessman B, Gilchrist S, Rose K, Spencer R, Hall J (Eds) (2017). Status Review, Disease Risk Analysis and Conservation Action Plan for the Bellinger River Snapping Turtle. IUCN SSC Conservation Breeding Specialist Group: Apple Valley, MN. Cover photo: Juvenile Bellinger River Snapping Turtle © 2016 Brett Vercoe This report can be downloaded from the CBSG website: www.cbsg.org. 2 Executive Summary The Bellinger River Snapping Turtle (BRST) (Myuchelys georgesi) is a freshwater turtle endemic to a 60 km stretch of the Bellinger River, and possibly a portion of the nearby Kalang River in coastal north eastern New South Wales (NSW). -

NSW Police Gazette 1878
This sampler file contains various sample pages from the product. Sample pages will often include: the title page, an index, and other pages of interest. This sample is fully searchable (read Search Tips) but is not FASTFIND enabled. To view more samplers click here www.gould.com.au www.archivecdbooks.com.au · The widest range of Australian, English, · Over 1600 rare Australian and New Zealand Irish, Scottish and European resources books on fully searchable CD-ROM · 11000 products to help with your research · Over 3000 worldwide · A complete range of Genealogy software · Including: Government and Police 5000 data CDs from numerous countries gazettes, Electoral Rolls, Post Office and Specialist Directories, War records, Regional Subscribe to our weekly email newsletter histories etc. FOLLOW US ON TWITTER AND FACEBOOK www.unlockthepast.com.au · Promoting History, Genealogy and Heritage in Australia and New Zealand · A major events resource · regional and major roadshows, seminars, conferences, expos · A major go-to site for resources www.familyphotobook.com.au · free information and content, www.worldvitalrecords.com.au newsletters and blogs, speaker · Free software download to create biographies, topic details · 50 million Australasian records professional looking personal photo books, · Includes a team of expert speakers, writers, · 1 billion records world wide calendars and more organisations and commercial partners · low subscriptions · FREE content daily and some permanently This sampler file includes the title page and various sample pages from this volume. This file is fully searchable (read search tips page) but is not FASTFIND enabled New South Wales Police Gazette 1878 Ref. AU2103-1878 ISBN: 978 1 921416 10 1 This book was kindly loaned to Archive CD Books Australia by New South Wales Justice and Police Museum http://www.hht.net.au/museums/jp/justice_and_police_museum Navigating this CD To view the contents of this CD use the bookmarks and Adobe Reader’s forward and back buttons to browse through the pages. -

Effects of Estuarine Acidification on Survival and Growth of the Sydney Rock Oyster Saccostrea Glomerata
EFFECTS OF ESTUARINE ACIDIFICATION ON SURVIVAL AND GROWTH OF THE SYDNEY ROCK OYSTER SACCOSTREA GLOMERATA Michael Colin Dove Submitted in fulfilment of the requirements of the degree of Doctor of Philosophy in The University of New South Wales Geography Program Faculty of the Built Environment The University of New South Wales Sydney, NSW, 2052 April 2003 ACKNOWLEDGEMENTS I would like to thank my supervisor Dr Jes Sammut for his ideas, guidance and encouragement throughout my candidature. I am indebted to Jes for his help with all stages of this thesis, for providing me with opportunities to present this research at conferences and for his friendship. I thank Dr Richard Callinan for his assistance with the histopathology and reviewing chapters of this thesis. I am also very grateful to Laurie Lardner and Ian and Rose Crisp for their invaluable advice, generosity and particular interest in this work. Hastings and Manning River oyster growers were supportive of this research. In particular, I would like to acknowledge the following oyster growers: Laurie and Fay Lardner; Ian and Rose Crisp; Robert Herbert; Nathan Herbert; Stuart Bale; Gary Ruprecht; Peter Clift; Mark Bulley; Chris Bulley; Bruce Fairhall; Neil Ellis; and, Paul Wilson. I am very grateful to Holiday Coast Oysters and Manning River Rock Oysters for providing: the Sydney rock oysters for field and laboratory experiments; storage facilities; equipment; materials; fuel; and, access to resources without reservation. Bruce Fairhall, Paul Wilson, Mark Bulley, Laurie Lardner and Robert Herbert also supplied Sydney rock oysters for this work. I would also like to thank the researchers who gave helpful advice during this study. -

Transport for NSW Mid-North Coast Regional Boating Plan
Transport for NSW Regional Boating Plan Mid-North Coast Region February 2015 Transport for NSW 18 Lee Street Chippendale NSW 2008 Postal address: PO Box K659 Haymarket NSW 1240 Internet: www.transport.nsw.gov.au Email: [email protected] ISBN Register: 978 -1 -922030 -68 -9 © COPYRIGHT STATE OF NSW THROUGH THESECRETARY OF TRANSPORT FOR NSW 2014 Extracts from this publication may be reproduced provided the source is fully acknowledged. Report for Transport for NSW - Regional Boating Plan| i Table of contents 1. Introduction..................................................................................................................................... 4 2. Physical character of the waterways .............................................................................................. 6 2.1 Background .......................................................................................................................... 6 2.2 Bellinger and Nambucca catchments and Coffs Harbour area ........................................... 7 2.3 Macleay catchment .............................................................................................................. 9 2.4 Hastings catchment ............................................................................................................. 9 2.5 Lord Howe Island ............................................................................................................... 11 2.6 Inland waterways .............................................................................................................. -

Bird Watching
ADVENTURE EXPERIENCES Parts of New England High Country belong to the East Kunderang Homestead offers World Heritage Gondwana Rainforests of Australia beautifully restored heritage accommodation for up to 14 people BOULDERING on unique rock formations; New England granite experience a multi-day bushwalking adventure with lots of crimps, flakes and through world-heritage national parks friction. Stonehenge Recreation Park near Glen Innes has over seventy routes. Gara The park’s rock formations are particularly Gorge near Armidale and Bald Rock near photogenic in late-afternoon light or mist. Tenterfield are other classic bouldering Hundreds of kilometres of waterways and rockclimbing destinations. NEW ENGLAND HIGH COUNTRY BOASTS rage and meander through the Oxley Wild Rivers National Park, where huge tracts AN EXTENSIVE GORGE WILDERNESS WITH OVER 500 of magnificent wilderness are also World KILOMETRES OF WILD AND SCENIC RIVERS. DISCOVER Heritage listed. This is where camping, trout MOUNTAIN BIKING is massive in fishing, kayaking, swimming, canyoning and East Kunderang Homestead New England High Country. Local A UNIQUE NATURAL PLAYGROUND WITH MAGNIFICENT abseiling are major attractions. clubs have made over 40km of WATERFALLS, RAINFORESTS AND EXOTIC FLORA AND FAUNA. tracks that present some spectacular and purpose-built tracks around Guyra and The 7km walk from Budds Mare to Riverside varied wilderness walking opportunities. An Armidale. At Washpool and Gibraltar you descends 700m to the Apsley River with great easy amble along the beautiful Coombadhja GREEN GULLY TRACK NEW ENGLAND can ride a 50km loop through World bass fishing, swimming holes, camping and WILDERNESS TRAIL Imagine a place where the geology and biodiversity is Creek takes you through rainforest and dry Experience fern-lined gullies, high Heritage National Parks. -

Introduction the Need for New Reserves
Koala Strategy Submissions, PO Box A290, Sydney South NSW 1232, [email protected] CC. [email protected] 2nd March 2017. NPA SUBMISSION: WHOLE OF GOVERNMENT KOALA STRATEGY; SAVING OUR SPECIES DRAFT STRATEGY AND REVIEW OF STATE ENVIRONMENT PLANNING POLICY 44—KOALA HABITAT PROTECTION Introduction The National Parks Association of NSW (NPA), established in 1957, is a community-based organisation with over 20,000 supporters from rural, remote and urban areas across the state. NPA promotes nature conservation and evidence- based natural resource management. We have a particular interest in the protection of the State’s biodiversity and supporting ecological processes, both within and outside of the formal conservation reserve system. NPA has a long history of engagement with both government and non-government organisations on issues of park management. NPA appreciates the opportunity to comment on the whole of government koala strategy (the strategy), the Saving Our Species iconic koala project (the SOS project) and the Explanation of intended effect: State Environment and Planning Policy 44 (Koala Habitat Protection) (SEPP 44). Please note, NPA made a submission on SEPP 44 in late 2016 prior to the deadline being extended which we have reattached at the end of this document (Appendix 1). NPA was also consulted on the SOS programme in August 2016, which we greatly appreciated. We provided OEH with feedback on the programme at that time, which also contained an exploration of issues facing koalas, which we have reattached in Appendix 2. NPA has had significant input to the development of the Stand Up For Nature (SUFN) submission to this consultation. -

Vicnews101 V2.Qxd
VICNEWS Number 109 August 2014 ANGFA Victoria Inc. is a regional group member of AUSTRALIA NEW GUINEA FISHES ASSOCIATION INC. Published by ANGFA Victoria Inc. PO Box 298, Chirnside Park Vic. 3116 Visit us at: www.angfavic.org and on Facebook Unravelling the Galaxias olidus species complex with Tarmo Raadik, our August meeting guest presenter Photo above: A fast flowing, pristine creek in the upper catch - ment zone of a Victorian high country river. These types of creeks are the natural habitat of upland galaxiids in the Mountain Galaxias (Galaxias olidus) species complex, with some species widespread and others confined to single, small catch - ments. This type of waterfall is often the key reason why the species survive, as their main predators, Brown Trout and Rainbow Trout, are unable to move further upstream. Many of the restricted species are also under a high risk of extinction from instream sedimentation following increasingly frequent wild fire. Photo: Tarmo Raadik Photo to right: Galaxias fuscus . Photo: Tarmo Raadik VICNEWS August 2013 PAGE 1 environments, including fish passage assessment and fish - way monitoring, environmental flow determinations, fauna assessments in degraded, pristine and remote locations, and alien species control. In particular, Tarmo has a long involvement with the compilation and management of aquatic fauna survey data, undertaking the first compilation of Victorian data in 1986, which he then continued while at the Department of Ichthyology, Museum Victoria, and later when he joined the Arthur Rylah Institute. This data compilation became the basis the new Victorian Biodiversity Atlas (VBA). Tarmo holds the roles of Taxon Manager and Data Expert Reviewer for Aquatic Fauna (fish, freshwater decapod crus - tacea and molluscs) for the VBA, and is relied upon by ARI staff and external clients for his knowledge of current tax - onomy and historical and contemporary aquatic fauna dis - tributions. -

Functioning and Changes in the Streamflow Generation of Catchments
Ecohydrology in space and time: functioning and changes in the streamflow generation of catchments Ralph Trancoso Bachelor Forest Engineering Masters Tropical Forests Sciences Masters Applied Geosciences A thesis submitted for the degree of Doctor of Philosophy at The University of Queensland in 2016 School of Earth and Environmental Sciences Trancoso, R. (2016) PhD Thesis, The University of Queensland Abstract Surface freshwater yield is a service provided by catchments, which cycle water intake by partitioning precipitation into evapotranspiration and streamflow. Streamflow generation is experiencing changes globally due to climate- and human-induced changes currently taking place in catchments. However, the direct attribution of streamflow changes to specific catchment modification processes is challenging because catchment functioning results from multiple interactions among distinct drivers (i.e., climate, soils, topography and vegetation). These drivers have coevolved until ecohydrological equilibrium is achieved between the water and energy fluxes. Therefore, the coevolution of catchment drivers and their spatial heterogeneity makes their functioning and response to changes unique and poses a challenge to expanding our ecohydrological knowledge. Addressing these problems is crucial to enabling sustainable water resource management and water supply for society and ecosystems. This thesis explores an extensive dataset of catchments situated along a climatic gradient in eastern Australia to understand the spatial and temporal variation -

Acid Sulfate Soil Induced Acidification of Estuarine Areas Used for the Production of Sydney Rock Oysters, Saccostrea Glomerata
Journal of Water Resource and Protection, 2013, 5, 320-335 http://dx.doi.org/10.4236/jwarp.2013.53A033 Published Online March 2013 (http://www.scirp.org/journal/jwarp) Acid Sulfate Soil Induced Acidification of Estuarine Areas Used for the Production of Sydney Rock Oysters, Saccostrea glomerata Michael C. Dove1*, Jesmond Sammut2 1Port Stephens Fisheries Centre, NSW Department of Primary Industries, Taylors Beach, Australia 2Australian Wetlands and Rivers Centre, School of Biological, Earth & Environmental Sciences, The University of New South Wales, Sydney, Australia Email: *[email protected] Received December 10, 2012; revised January 10, 2013; accepted January 18, 2013 ABSTRACT This study investigated estuarine acidification, associated with drainage and excavation of acid sulfate soils, in areas used for commercial cultivation of Sydney rock oysters (Saccostrea glomerata). Regular measurements of pH and elec- trical conductivity were collected in oyster cultivation areas and acidified reaches of the Hastings River estuary and Port Stephens estuary located on the mid north coast of New South Wales, Australia. Water quality information from acidi- fied floodplain drains was also collected in the Hastings River following heavy rainfall. Both estuaries experienced acidification of tributaries following periods of heavy rainfall. Drain outflow waters were acidic (pH < 3.5); contained elevated concentrations of iron, aluminium, manganese and zinc; and polluted areas used for oyster production. The extent and duration of estuarine acidification events was capable of causing a variety of short- and long-term impacts to oysters as well as other aquatic organisms in affected areas. Keywords: Estuarine Acidification; Acid Sulfate Soil; Sydney Rock Oyster; Saccostrea glomerata; New South Wales 1. -

Kalang River Health Plan
KALANG RIVER HEALTH PLAN BellingenBelligen ShireShire CouncilCouncil Bellingen Shire Healthy Rivers Program Kalang River Health Plan Title Kalang River Health Plan Date of Publication March 2010 Copyright Copyright © 2010, Bellingen Shire Council. Along with any use permitted under the Copyright Act 1968, this report may also be reproduced in part or whole for the purposes of study, research, review and/or general information provided clear acknowledgement of the source is made. Citation Bellingen Shire Council (2010). Kalang River Health Plan. Bellingen Shire Council, NSW. ISBN 978 0 9807685 2 7 (Hard copy) ISBN 978 0 9807685 3 4 (Digital copy) Digital Copy Access This report can be accessed and downloaded on line at www.bellingen.nsw.gov.au by following the Environment prompt to the Healthy Rivers Program. Disclaimer While every care has been taken to ensure that the information contained in this report is accurate, complete and current at the time of publication, no warranty is given or implied that it is free of error or omission. No liability is accepted for any material or financial loss, damage, cost or injury arising from the use of this report. Funding Support This report has been prepared by Bellingen Shire Council with financial assistance from the NSW Government through the Department of Environment, Climate Change and Water. This document does not necessarily reflect the opinions of the NSW Government or the Department of Environment, Climate Change and Water. Cover Image Kalang River estuary Bellingen Shire Healthy Rivers Program Kalang River Health Plan KALANG RIVER HEALTH PLAN KALANG RIVER HEALTH PLAN Foreword Kalang River Health Plan Our coastal waterways are under increasing pressure from population growth and change to the traditional low impact industries.