Acid Sulfate Soil Induced Acidification of Estuarine Areas Used for the Production of Sydney Rock Oysters, Saccostrea Glomerata
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
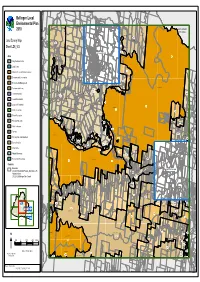
LEP 2010 LZN Template
WILD CATTLE CREEK E1E1E1 DORRIGO RU2RU2 GLENREAGH MULDIVA RU2RU2 OLD BILLINGS RD RAILWAY NATURE COAST TYRINGHAM RESERVE RD RD Bellingen Local E1EE1E11 BORRA CREEK DORRIGO NATIONAL PARK CORAMBA RD E3EE3E33 SLINGSBYS Environmental Plan LITTLE MURRAY RIVER RU2RRU2U2 RD LITTLE PLAIN CREEK BORRA CREEK TYRINGHAM COFFSCOFFS HARBOURHARBOUR 2010 RD CITYCCITYITY COUNCILCOUNCIL BREAKWELLS WILD CATTLE CREEK RD RU2RRU2U2 CORAMBA RD RU2RU2 NEAVES NEAVES RD RD SLINGSBYS RD Land Zoning Map LITTLE MURRAY RIVER OLD COAST RD E3EE3E33 RU2RRU2U2 Sheet LZN_004 E3EE3E33 DEER VALE RD OLD CORAMBA TYRINGHAM RD NTH RD ROCKY CREEK RU1RU1 BARTLETTS RD ReferRefer toto mapmap LZN_004ALZN_004A Zone RU1RRU1U1 BIELSDOWN RIVER E1EE1E11 DEER VALE RD B1 Neighbourhood Centre RU1RU1 DORRIGO NATIONAL PARK B2 Local Centre JOHNSENS WATERFALL WAY OLD CORAMBA WATERFALL WAY RD STH EE3E3E33 E1 National Parks and Nature Reserves E3E3E3 BENNETTS RD E2 Environmental Conservation WATERFALL WAY SHEPHERDS RD RU1RRU1U1 E3 Environmental Management DOME RD EVERINGHAMS ROCKY CREEK RD BIELSDOWN RIVER DORRIGO NATIONAL PARK E4 Environmental Living E3EE3E33 SP1SP1SP1 E3E3E3 SHEPHERDS RD WHISKY CEMETERYCCEMETERYEMETERY DOME RD IN1 General Industrial CREEK RD RU1RRU1U1 RU1RRU1U1 WOODLANDS RD WATERFALL R1 General Residential WAY RU1RRU1U1 PROMISED RU1RU1 E3E3E3 LAND RD WATERFALL WAY R5 Large Lot Residential WHISKY CREEK E1EE1E11 RU1RU1 DORRIGO NATIONAL PARK E1E1E1 E1EE1E11 RE1 Public Recreation BIELSDOWN RIVER E3EE3E33 ROCKY CREEK RD RE2 Private Recreation WHISKY CREEK RD RU1RRU1U1 RU2RU2 E3E3E3 -

Estuary Surveillance for QX Disease
Estuary surveillance Student task sheet for QX disease The following tables show data collected Estuary Surveillance 2002: during estuary surveillance from 2001– During the 2002 sampling period a total of 2004 for New South Wales and 5250 oysters were received and processed Queensland. N is the number of oysters from 18 NSW estuaries and three tested in a random sample of the oyster Queensland zones using tissue imprints. population. Dr Adlard used two methods of disease detection in surveillance — tissue imprint and PCR. Table 2A: Tissue imprints used to detect the QX disease parasite Estuary Surveillance 2001: 2002 Survey results Table 1: Tissue imprint results for 2001 N 2001 Survey Results Estuary N infected % N Northern Moreton Bay 250 0 0 Estuary N infected % Central Moreton Bay 250 0 0 Tweed River 316 0 0 Southern Moreton Bay 250 2 0.8 Brunswick River 320 0 0 Tweed River 250 0 0 Richmond River 248 0 0 Brunswick River 250 0 0 Clarence River 330 5 1.52 Richmond River 250 102 40.8 Wooli River 294 0 0 Clarence River 250 55 22 Kalang /Bellinger 295 0 0 Wooli River 250 0 0 Rivers Kalang /Bellingen Rivers 250 0 0 Macleay River 261 0 0 Macleay River 250 0 0 Hastings River 330 0 0 Hastings River 250 0 0 Manning River 286 0 0 Manning River 250 0 0 Wallis Lakes 271 0 0 Wallis Lakes 250 0 0 Port Stephens 263 0 0 Port Stephens 250 0 0 Hawkesbury River 323 0 0 Hawkesbury River 250 0 0 Georges River 260 123 47.31 Georges River 250 40 16 Shoalhaven/ 255 0 0 Crookhaven Shoalhaven/Crookhaven 250 0 0 Bateman's Bay 300 0 0 Bateman's Bay 250 0 0 Tuross Lake 304 0 0 Tuross Lake 250 0 0 Narooma 300 0 0 Narooma 250 0 0 Merimbula 250 0 0 Merimbula 250 0 0 © Queensland Museum 2006 Table 2B: PCR results from 2002 on Estuary Surveillance 2003: oysters which had tested negative to QX During 2003 a total of 4450 oysters were disease parasite using tissue imprints received and processed from 22 NSW estuaries and three Queensland zones. -

Management of Acid Sulfate Soils by Groundwater Manipulation Bruce Geoffrey Blunden University of Wollongong
University of Wollongong Research Online University of Wollongong Thesis Collection University of Wollongong Thesis Collections 2000 Management of acid sulfate soils by groundwater manipulation Bruce Geoffrey Blunden University of Wollongong Recommended Citation Blunden, Bruce Geoffrey, Management of acid sulfate soils by groundwater manipulation, Doctor of Philosophy thesis, Faculty of Engineering, University of Wollongong, 2000. http://ro.uow.edu.au/theses/1834 Research Online is the open access institutional repository for the University of Wollongong. For further information contact the UOW Library: [email protected] MANAGEMENT OF ACID SULFATE SOILS BY GROUNDWATER MANIPULATION A thesis submitted in fulfilment of the requirements of the degree of DOCTOR OF PHILOSOPHY from UNIVERSITY OF WOLLONGONG by BRUCE GEOFFREY BLUNDEN B.Nat Res. (Hons), M.Eng. Faculty of Engineering October 2000 AFFIRMATION I, Bruce Blunden, declare that this thesis, submitted in fulfilment of the requirements for the award of Doctor of Philosophy, in the Faculty of Engineering, University of Wollongong, is wholly my own work unless otherwise referenced or acknowledged. This document has not been submitted for qualifications at any other academic institution. Bruce Blunden. n PUBLICATIONS RELATED TO THIS RESEARCH Blunden, B. and Indraratna, B. (2000). Evaluation of Surface and Groundwater Management Strategies for Drained Sulfidic Soil using Numerical Models. Australian Journal of Soil Research 38, 569-590 Blunden, B. and Indraratna, B. (2000). Pyrite Oxidation Model for Assessing Groundwater management Strategies in Acid Sulphate Soils. Journal of Geotechnical and Geoenvironmental Engineering, (in press). Indraratna, B. and Blunden, B. (1999). Nature and Properties of Acid Sulphate Soils in Drained Coastal lowland in NSW, Australian Geomechanics Journal, 34(1), 61-78. -

Ordinary Council Meeting Agenda Items 25 March 2021
NAMBUCCA VALLEY COUNCIL ORDINARY COUNCIL MEE TING AGENDA ITEMS 25 MARCH 2021 Council has adopted the following Vision and Mission Statements to describe its philosophy and to provide a focus for the principal activities detailed in its Management Plan. Our Vision Nambucca Valley ~ Living at its best. Our Mission Statement ‘The Nambucca Valley will value and protect its natural environment, maintain its assets and infrastructure and develop opportunities for its people.’ Our Values in Delivery Effective leadership Strategic direction Sustainability of infrastructure and assets Community involvement and enhancement through partnerships with Council Enhancement and protection of the environment Maximising business and employment opportunities through promotion of economic development Addressing social and cultural needs of the community through partnerships and provision of facilities and services Actively pursuing resource sharing opportunities Council Meetings: Overview and Proceedings Council meetings are held on the last Thursday of each month AND on the Thursday two weeks before the Thursday meeting. Both meetings commence at 5.30 pm. Meetings are held in the Council Chamber at Council's Administration Centre—44 Princess Street, Macksville (unless otherwise advertised). How can a Member of the Public Speak at a Council Meeting? 1 Addressing Council with regard to an item on the meeting agenda: Members of the public are welcome to attend meetings and address the Council. Registration to speak may be made by telephone or in person before 11.00 am on a meeting day. The relevant agenda item will be brought forward at 5.30 pm in agenda order, and dealt with following preliminary business items on the agenda. -

Bellinger and Kalang River Estuaries Erosion Study
Bellinger and Kalang River Estuaries Erosion Study Damon Telfer GECO Environmental Tim Cohen IRM Consultants February 2010 Report prep ared for BELLINGEN SHIRE COUNCIL DISCLAIMER Whilst every care has been taken in compiling this erosion study and report, no assurances are given that it is free from error or omission. The authors make no expressed or implied warranty of the accuracy or fitness of the recommendations in the report and disclaim all liability for the consequences of anything done or omitted to be done by any person in reliance upon these recommendations. The use of this report is at the user’s risk and discretion. The authors will not be liable for any loss, damage, costs or injury including consequential, incidental, or financial loss arising out of the use of this report. This report was produced with financial assistance from the NSW Government through the Department of Environment, Climate Change and Water. This document does not necessarily represent the opinions of the NSW Government or the Department of Environment, Climate Change and Water. Damon Telfer GECO Environmental 5 Arcadia Lane, GRASSY HEAD NSW 2441 Email: [email protected] © Damon Telfer, February 2010. This document is copyright and cannot be reproduced in part or whole without the express permission of the author. A permanent, irrevocable royalty-free, non-exclusive license to make these reports, documents and any other materials publicly available and to otherwise communicate, reproduce, adapt and publicise them on a non- profit basis is granted by the authors to Bellingen Shire Council and the Department of Environment, Climate Change and Water. -

Wabbra Squattage of Betts and Panton on the Macleay River – 32,000 Acres
W Wabbra Squattage of Betts and Panton on the Macleay river – 32,000 acres. (see History of Port Macquarie, p.117). Waddys Farm Rosewood (see PMN, 27 aug 1910 p.4). Wade Park A public reserve located in Beechwood. Wade is an old name in Gazetted: 5 Feb 1993 the district. Wade Street Wauchope. Named for Monie and Flo Wade. Wademont Property of A. Wade at Mile Scrub near Rollands Plains August 1902. Waitui Waterfall A waterfall on Stewarts River about 5km W by S of Dellward & Gazetted: 26 Jun 1987 about 8km NW of Hannam Vale and 12km SW of Lorne. Walgett No. 1 Property 3km N of Kendall. Walgett No. 2 Property 3km N of Kendall. Walhalla Farm on the Wauchope Comboyne Road near the junction of Jones Road - 3km E of Byabarra. Walkers Creek A partly perennial stream rising 2km SSW of town of Herons Gazetted: 26 Jun 1987 Creek flows for about 3km towards Herons Creek. The lower course is indefinite in parts. Walkham Vale Farm on the Forbes River 3km NW of Birdwood. Wall A reserve beside the training wall at North Haven about 1km NE Reserve of Ostler Park. Gazetted: 9 Jan 1987 Wall Street North Haven. The road leading to the break wall. Wallaby Creek Road Runs SE off The Scrub Road 6km W of Wauchope. Wallaby Hill "Wallaby Hill lies between Willsbro' and Glen Esk, about three miles from each". (see Empire 2nd March 1871, p.4). Original grant of Major Innes then to Rev. T. O’Reilly - Mr and Mrs Stewart – J.T.Guihen 13 May 1899. -

THE REGIONAL APPROACH NSW Local Health Districts Mid North Coast Local Health District Local Government Areas
ENVIRONMENTAL HEALTH “NOT IN ISOLATION” THE REGIONAL APPROACH NSW Local Health Districts Mid North Coast Local Health District Local Government Areas ∗ Port Macquarie-Hastings Council ∗ Kempsey Shire Council ∗ Nambucca Shire Council ∗ Bellingen Shire Council ∗ Coffs Harbour City Council Resources ∗ There are around 24 staff in the region between both Councils and the Ministry ∗ Ministry staff are exclusive EHO’s ∗ The larger Councils have specific EHO’s that rotate through functions ∗ The smaller Councils traditionally have Health and Building surveyors that cover the facets of EH and Building and land use related issues David Basso (EHO) EHA NSW MNC Regional Group ∗ The EHA (NSW)Inc. Mid North Coast regional group has been meeting now for almost 20 years ∗ The quarterly meetings of the group have given rise to a range of joint region-wide surveys, seminars, inspections etc. for Regulated Environmental Health functions Never short of issues ∗ Matters of a strategic and reactive nature include; ∗ Rave parties – Bellingen and Coffs ∗ Drug labs ∗ Floods ∗ Bellinger River snapping turtle ∗ Abandoned Antimony processing site – Urunga ∗ Sewerage overflows ∗ Oyster Aquaculture ∗ Water Recycling ∗ Diesel spills and truck rollovers Scheduled activities ∗ Skin Penetration ∗ Public swimming pools ∗ Private drinking water supplies ∗ On site sewerage management ∗ Food Businesses ∗ Mosquito vector program ∗ Legionella control Aboriginal Environmental Health ∗ The Ministry of Health employ Aboriginal Trainee EHOs – some positions shared with regional councils ∗ This facilitates outcomes in Aboriginal communities and good range of experiences ∗ A good example of programs that the Ministry are involved with include ‘Housing for Health’ Across The Region # Food business premises – 1387 # OSSM – 22806 # Public swimming pools – 227 # Private Swimming pools – 5120 # Skin Penetration premises – 126 # Cooling towers/warm water systems – 40+ # Brothels – upward of 12 # Water carters – 30+ OYSTER AQUACULTURE ∗ River closures by the NSW Food Authority have caused stress on local industry. -

Ocean Acidification Habitat Committee, It Is My Pleasure to Present the 2014 Habitat Effects on Atlantic Coral Reefs
HABITATHABITAT HOTLINEHOTLINE Atlantic 2014 Annual Issue HEALTHY FISHERIES NEED HEALTHY HABITAT In This Issue Climate Change Impacts How Climate Change Affects Species Range and Habitats ..................... 2 on Fish Habitats Climate Data Collection Efforts Supporting Fisheries Management in the Northeast ......................................... 3 As the Chair of the Atlantic States Marine Fisheries Commission’s Ocean Acidification Habitat Committee, it is my pleasure to present the 2014 Habitat Effects on Atlantic Coral Reefs ..........................4 Hotline Atlantic. This year’s issue explores some of the processes Slowly Dissolving Shellfish .................................5 Effects on Planktonic Resources .......................5 and impacts of climate change on marine and estuarine fish habitats along the United States’ East Coast. According to EPA’s Climate Sea Level Rise and Its Effects Change Indicators in the United States, 2014, climate change refers to Coastal Salt Marshes ...............................................6 Oyster Reefs ................................................................7 “any substantial change in measure of climate lasting for an extended Salt Marshes and Mangroves ................................8 period (decades or longer) that may result from natural factors and Habitat Management ................................ 8 process from human activities.” Some of these changes consist of Considerations and Tools for Adaptation increased ocean and sea surface temperatures, sea level rise, and to -

Fluvial Geomorphology of the Nambucca River Catchment: Late Quaternary Change, Post-Settlement Channel Degradation and Proposals for Rehabilitation Christopher J
University of Wollongong Research Online University of Wollongong Thesis Collection University of Wollongong Thesis Collections 2003 Fluvial geomorphology of the Nambucca river catchment: late quaternary change, post-settlement channel degradation and proposals for rehabilitation Christopher J. Doyle University of Wollongong Recommended Citation Doyle, Christopher J., Fluvial geomorphology of the Nambucca river catchment: late quaternary change, post-settlement channel degradation and proposals for rehabilitation, Doctor of Philosophy thesis, School of Geosciences, University of Wollongong, 2003. http://ro.uow.edu.au/theses/1969 Research Online is the open access institutional repository for the University of Wollongong. For further information contact the UOW Library: [email protected] NOTE This online version of the thesis may have different page formatting and pagination from the paper copy held in the University of Wollongong Library. UNIVERSITY OF WOLLONGONG COPYRIGHT WARNING You may print or download ONE copy of this document for the purpose of your own research or study. The University does not authorise you to copy, communicate or otherwise make available electronically to any other person any copyright material contained on this site. You are reminded of the following: Copyright owners are entitled to take legal action against persons who infringe their copyright. A reproduction of material that is protected by copyright may be a copyright infringement. A court may impose penalties and award damages in relation to offences and infringements relating to copyright material. Higher penalties may apply, and higher damages may be awarded, for offences and infringements involving the conversion of material into digital or electronic form. FLUVIAL GEOMORPHOLOGY OF THE NAMBUCCA RIVER CATCHMENT: LATE QUATERNARY CHANGE, POST-SETTLEMENT CHANNEL DEGRADATION AND PROPOSALS FOR REHABILITATION A thesis submitted in fulfilment of the requirements for the award of the degree DOCTOR OF PHILOSOPHY from University of Wollongong by Christopher J. -

Status Review, Disease Risk Analysis and Conservation Action Plan for The
Status Review, Disease Risk Analysis and Conservation Action Plan for the Bellinger River Snapping Turtle (Myuchelys georgesi) December, 2016 1 Workshop participants. Back row (l to r): Ricky Spencer, Bruce Chessman, Kristen Petrov, Caroline Lees, Gerald Kuchling, Jane Hall, Gerry McGilvray, Shane Ruming, Karrie Rose, Larry Vogelnest, Arthur Georges; Front row (l to r) Michael McFadden, Adam Skidmore, Sam Gilchrist, Bruno Ferronato, Richard Jakob-Hoff © Copyright 2017 CBSG IUCN encourages meetings, workshops and other fora for the consideration and analysis of issues related to conservation, and believes that reports of these meetings are most useful when broadly disseminated. The opinions and views expressed by the authors may not necessarily reflect the formal policies of IUCN, its Commissions, its Secretariat or its members. The designation of geographical entities in this book, and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of IUCN concerning the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. Jakob-Hoff, R. Lees C. M., McGilvray G, Ruming S, Chessman B, Gilchrist S, Rose K, Spencer R, Hall J (Eds) (2017). Status Review, Disease Risk Analysis and Conservation Action Plan for the Bellinger River Snapping Turtle. IUCN SSC Conservation Breeding Specialist Group: Apple Valley, MN. Cover photo: Juvenile Bellinger River Snapping Turtle © 2016 Brett Vercoe This report can be downloaded from the CBSG website: www.cbsg.org. 2 Executive Summary The Bellinger River Snapping Turtle (BRST) (Myuchelys georgesi) is a freshwater turtle endemic to a 60 km stretch of the Bellinger River, and possibly a portion of the nearby Kalang River in coastal north eastern New South Wales (NSW). -

(Phascolarctos Cinereus) on the North Coast of New South Wales
A Blueprint for a Comprehensive Reserve System for Koalas (Phascolarctos cinereus) on the North Coast of New South Wales Ashley Love (President, NPA Coffs Harbour Branch) & Dr. Oisín Sweeney (Science Officer, NPA NSW) April 2015 1 Acknowledgements This proposal incorporates material that has been the subject of years of work by various individuals and organisations on the NSW north coast, including the Bellengen Environment Centre; the Clarence Environment Centre; the Nambucca Valley Conservation Association Inc., the North Coast Environment Council and the North East Forest Alliance. 2 Traditional owners The NPA acknowledges the traditional Aboriginal owners and original custodians of the land mentioned in this proposal. The proposal seeks to protect country in the tribal lands of the Bundjalung, Gumbainggir, Dainggatti, Biripi and Worimi people. Citation This document should be cited as follows: Love, Ashley & Sweeney, Oisín F. 2015. A Blueprint for a comprehensive reserve system for koalas (Phascolarctos cinereus) on the North Coast of New South Wales. National Parks Association of New South Wales, Sydney. 3 Table of Contents Acknowledgements ....................................................................................................................................... 2 Traditional owners ........................................................................................................................................ 3 Citation ......................................................................................................................................................... -

Effects of Estuarine Acidification on an Oyster-Associated Community in New South Wales, Australia
W&M ScholarWorks VIMS Articles Virginia Institute of Marine Science 2018 Effects Of Estuarine Acidification On An Oyster-Associated Community In New South Wales, Australia Cassandra N. Glaspie Virginia Institute of Marine Science SR Jenkinson RD Seitz Virginia Institute of Marine Science Follow this and additional works at: https://scholarworks.wm.edu/vimsarticles Part of the Marine Biology Commons Recommended Citation Glaspie, Cassandra N.; Jenkinson, SR; and Seitz, RD, "Effects Of Estuarine Acidification On An Oyster- Associated Community In New South Wales, Australia" (2018). VIMS Articles. 298. https://scholarworks.wm.edu/vimsarticles/298 This Article is brought to you for free and open access by the Virginia Institute of Marine Science at W&M ScholarWorks. It has been accepted for inclusion in VIMS Articles by an authorized administrator of W&M ScholarWorks. For more information, please contact [email protected]. Journal of Shellfish Research, Vol. 37, No. 1, 63–72, 2018. EFFECTS OF ESTUARINE ACIDIFICATION ON AN OYSTER-ASSOCIATED COMMUNITY IN NEW SOUTH WALES, AUSTRALIA CASSANDRA N. GLASPIE,1*† SARAH R. JENKINSON2 AND ROCHELLE D. SEITZ1 1Department of Biological Sciences, Virginia Institute of Marine Science, College of William & Mary, Gloucester Point, Virginia 23062; 2Department of Biological Sciences, Macquarie University, North Ryde, Sydney, New South Wales 2109, Australia ABSTRACT Many of the features that make estuaries among the most productive natural systems on earth also make them prone to acidification. Understanding the effects of estuarine acidification on different components of an ecological community is an important step in identifying indicators of ecosystem degradation. This study examined the impact of estuarine acidification, as a result of acid sulfate soil runoff, on wild Sydney rock oysters Saccostrea glomerata and their associated epifaunal communities in estuaries experiencing acid sulfate soil runoff in New South Wales, Australia.