Phylogenetic Constraints Upon Morphological and Ecological Adaptation in Hummingbirds (Trochilidae): Why Are There No Hermits in the Paramo?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Topazes and Hermits
Trochilidae I: Topazes and Hermits Fiery Topaz, Topaza pyra Topazini Crimson Topaz, Topaza pella Florisuginae White-necked Jacobin, Florisuga mellivora Florisugini Black Jacobin, Florisuga fusca White-tipped Sicklebill, Eutoxeres aquila Eutoxerini Buff-tailed Sicklebill, Eutoxeres condamini Saw-billed Hermit, Ramphodon naevius Bronzy Hermit, Glaucis aeneus Phaethornithinae Rufous-breasted Hermit, Glaucis hirsutus ?Hook-billed Hermit, Glaucis dohrnii Threnetes ruckeri Phaethornithini Band-tailed Barbthroat, Pale-tailed Barbthroat, Threnetes leucurus ?Sooty Barbthroat, Threnetes niger ?Broad-tipped Hermit, Anopetia gounellei White-bearded Hermit, Phaethornis hispidus Tawny-bellied Hermit, Phaethornis syrmatophorus Mexican Hermit, Phaethornis mexicanus Long-billed Hermit, Phaethornis longirostris Green Hermit, Phaethornis guy White-whiskered Hermit, Phaethornis yaruqui Great-billed Hermit, Phaethornis malaris Long-tailed Hermit, Phaethornis superciliosus Straight-billed Hermit, Phaethornis bourcieri Koepcke’s Hermit, Phaethornis koepckeae Needle-billed Hermit, Phaethornis philippii Buff-bellied Hermit, Phaethornis subochraceus Scale-throated Hermit, Phaethornis eurynome Sooty-capped Hermit, Phaethornis augusti Planalto Hermit, Phaethornis pretrei Pale-bellied Hermit, Phaethornis anthophilus Stripe-throated Hermit, Phaethornis striigularis Gray-chinned Hermit, Phaethornis griseogularis Black-throated Hermit, Phaethornis atrimentalis Reddish Hermit, Phaethornis ruber ?White-browed Hermit, Phaethornis stuarti ?Dusky-throated Hermit, Phaethornis squalidus Streak-throated Hermit, Phaethornis rupurumii Cinnamon-throated Hermit, Phaethornis nattereri Little Hermit, Phaethornis longuemareus ?Tapajos Hermit, Phaethornis aethopygus ?Minute Hermit, Phaethornis idaliae Polytminae: Mangos Lesbiini: Coquettes Lesbiinae Coeligenini: Brilliants Patagonini: Giant Hummingbird Lampornithini: Mountain-Gems Tro chilinae Mellisugini: Bees Cynanthini: Emeralds Trochilini: Amazilias Source: McGuire et al. (2014).. -

The Best of Costa Rica March 19–31, 2019
THE BEST OF COSTA RICA MARCH 19–31, 2019 Buffy-crowned Wood-Partridge © David Ascanio LEADERS: DAVID ASCANIO & MAURICIO CHINCHILLA LIST COMPILED BY: DAVID ASCANIO VICTOR EMANUEL NATURE TOURS, INC. 2525 WALLINGWOOD DRIVE, SUITE 1003 AUSTIN, TEXAS 78746 WWW.VENTBIRD.COM THE BEST OF COSTA RICA March 19–31, 2019 By David Ascanio Photo album: https://www.flickr.com/photos/davidascanio/albums/72157706650233041 It’s about 02:00 AM in San José, and we are listening to the widespread and ubiquitous Clay-colored Robin singing outside our hotel windows. Yet, it was still too early to experience the real explosion of bird song, which usually happens after dawn. Then, after 05:30 AM, the chorus started when a vocal Great Kiskadee broke the morning silence, followed by the scratchy notes of two Hoffmann´s Woodpeckers, a nesting pair of Inca Doves, the ascending and monotonous song of the Yellow-bellied Elaenia, and the cacophony of an (apparently!) engaged pair of Rufous-naped Wrens. This was indeed a warm welcome to magical Costa Rica! To complement the first morning of birding, two boreal migrants, Baltimore Orioles and a Tennessee Warbler, joined the bird feast just outside the hotel area. Broad-billed Motmot . Photo: D. Ascanio © Victor Emanuel Nature Tours 2 The Best of Costa Rica, 2019 After breakfast, we drove towards the volcanic ring of Costa Rica. Circling the slope of Poas volcano, we eventually reached the inspiring Bosque de Paz. With its hummingbird feeders and trails transecting a beautiful moss-covered forest, this lodge offered us the opportunity to see one of Costa Rica´s most difficult-to-see Grallaridae, the Scaled Antpitta. -

Volume 2. Animals
AC20 Doc. 8.5 Annex (English only/Seulement en anglais/Únicamente en inglés) REVIEW OF SIGNIFICANT TRADE ANALYSIS OF TRADE TRENDS WITH NOTES ON THE CONSERVATION STATUS OF SELECTED SPECIES Volume 2. Animals Prepared for the CITES Animals Committee, CITES Secretariat by the United Nations Environment Programme World Conservation Monitoring Centre JANUARY 2004 AC20 Doc. 8.5 – p. 3 Prepared and produced by: UNEP World Conservation Monitoring Centre, Cambridge, UK UNEP WORLD CONSERVATION MONITORING CENTRE (UNEP-WCMC) www.unep-wcmc.org The UNEP World Conservation Monitoring Centre is the biodiversity assessment and policy implementation arm of the United Nations Environment Programme, the world’s foremost intergovernmental environmental organisation. UNEP-WCMC aims to help decision-makers recognise the value of biodiversity to people everywhere, and to apply this knowledge to all that they do. The Centre’s challenge is to transform complex data into policy-relevant information, to build tools and systems for analysis and integration, and to support the needs of nations and the international community as they engage in joint programmes of action. UNEP-WCMC provides objective, scientifically rigorous products and services that include ecosystem assessments, support for implementation of environmental agreements, regional and global biodiversity information, research on threats and impacts, and development of future scenarios for the living world. Prepared for: The CITES Secretariat, Geneva A contribution to UNEP - The United Nations Environment Programme Printed by: UNEP World Conservation Monitoring Centre 219 Huntingdon Road, Cambridge CB3 0DL, UK © Copyright: UNEP World Conservation Monitoring Centre/CITES Secretariat The contents of this report do not necessarily reflect the views or policies of UNEP or contributory organisations. -

Appendix, French Names, Supplement
685 APPENDIX Part 1. Speciesreported from the A.O.U. Check-list area with insufficient evidencefor placementon the main list. Specieson this list havebeen reported (published) as occurring in the geographicarea coveredby this Check-list.However, their occurrenceis considered hypotheticalfor one of more of the following reasons: 1. Physicalevidence for their presence(e.g., specimen,photograph, video-tape, audio- recording)is lacking,of disputedorigin, or unknown.See the Prefacefor furtherdiscussion. 2. The naturaloccurrence (unrestrained by humans)of the speciesis disputed. 3. An introducedpopulation has failed to becomeestablished. 4. Inclusionin previouseditions of the Check-listwas basedexclusively on recordsfrom Greenland, which is now outside the A.O.U. Check-list area. Phoebastria irrorata (Salvin). Waved Albatross. Diornedeairrorata Salvin, 1883, Proc. Zool. Soc. London, p. 430. (Callao Bay, Peru.) This speciesbreeds on Hood Island in the Galapagosand on Isla de la Plata off Ecuador, and rangesat seaalong the coastsof Ecuadorand Peru. A specimenwas takenjust outside the North American area at Octavia Rocks, Colombia, near the Panama-Colombiaboundary (8 March 1941, R. C. Murphy). There are sight reportsfrom Panama,west of Pitias Bay, Dari6n, 26 February1941 (Ridgely 1976), and southwestof the Pearl Islands,27 September 1964. Also known as GalapagosAlbatross. ThalassarchechrysosWma (Forster). Gray-headed Albatross. Diornedeachrysostorna J. R. Forster,1785, M6m. Math. Phys. Acad. Sci. Paris 10: 571, pl. 14. (voisinagedu cerclepolaire antarctique & dansl'Ocean Pacifique= Isla de los Estados[= StatenIsland], off Tierra del Fuego.) This speciesbreeds on islandsoff CapeHorn, in the SouthAtlantic, in the southernIndian Ocean,and off New Zealand.Reports from Oregon(mouth of the ColumbiaRiver), California (coastnear Golden Gate), and Panama(Bay of Chiriqu0 are unsatisfactory(see A.O.U. -

Placement Student's Final Report*
Placement Student’s Final Report* LAURA CANNON *Abridged from original. Contains only Scientific Report portion. SCIENTIFIC REPORT THE EFFECT OF AN ARTIFICAL FEEDER ON HUMMINGBIRD BEHAVIOUR ABSTRACT Like their fellow nectavores, Hummingbirds try to maximize the ratio of energy obtained to energy expended; they try to consume as much nectar with as little movement as they can (Feinsinger, 1978; Hainsworth & Wolf, 1972). The way in which they optimize their consumption is through their ecologically defined roles, they behave in such a way to increase their chances of obtaining food; they often match the length of their bill to the length of the flower. Montgomerie & Gass (1981) noted that Hummingbirds abundance appears to be limited by the availability of nectar. In this study the presence of the artificial feeder provides a constant supply of food while it is up, which could cause a change in the Hummingbirds behaviour for example are maurders (bare in and feed) seen to convert to trapliners (visit multiple flowers), or territorialists (defenders) to become filchers (thieves)? Artificial feeders can affect hummingbirds foraging behaviour and abundance, but despite its potential importance, few previous studies have focused on the consequences of nectar feeders giving contradictory results (Feinsinger, 1978; Sonne et al., 2015). In a study by Arizmendi et al., (2007) the presence of a nectar feeder reduced hummingbird visitation to nearby plants in their study in Mexico. However in Brockmeyer and Schaefer’s study (2012) the number of visits to the flowers around the feeder increased when the feeder was present. This studies main focus is to determine how the presence of an artificial feeder affects the hummingbird’s behaviour specifically feeding, perching and aggression. -

Alpha Codes for 2168 Bird Species (And 113 Non-Species Taxa) in Accordance with the 62Nd AOU Supplement (2021), Sorted Taxonomically
Four-letter (English Name) and Six-letter (Scientific Name) Alpha Codes for 2168 Bird Species (and 113 Non-Species Taxa) in accordance with the 62nd AOU Supplement (2021), sorted taxonomically Prepared by Peter Pyle and David F. DeSante The Institute for Bird Populations www.birdpop.org ENGLISH NAME 4-LETTER CODE SCIENTIFIC NAME 6-LETTER CODE Highland Tinamou HITI Nothocercus bonapartei NOTBON Great Tinamou GRTI Tinamus major TINMAJ Little Tinamou LITI Crypturellus soui CRYSOU Thicket Tinamou THTI Crypturellus cinnamomeus CRYCIN Slaty-breasted Tinamou SBTI Crypturellus boucardi CRYBOU Choco Tinamou CHTI Crypturellus kerriae CRYKER White-faced Whistling-Duck WFWD Dendrocygna viduata DENVID Black-bellied Whistling-Duck BBWD Dendrocygna autumnalis DENAUT West Indian Whistling-Duck WIWD Dendrocygna arborea DENARB Fulvous Whistling-Duck FUWD Dendrocygna bicolor DENBIC Emperor Goose EMGO Anser canagicus ANSCAN Snow Goose SNGO Anser caerulescens ANSCAE + Lesser Snow Goose White-morph LSGW Anser caerulescens caerulescens ANSCCA + Lesser Snow Goose Intermediate-morph LSGI Anser caerulescens caerulescens ANSCCA + Lesser Snow Goose Blue-morph LSGB Anser caerulescens caerulescens ANSCCA + Greater Snow Goose White-morph GSGW Anser caerulescens atlantica ANSCAT + Greater Snow Goose Intermediate-morph GSGI Anser caerulescens atlantica ANSCAT + Greater Snow Goose Blue-morph GSGB Anser caerulescens atlantica ANSCAT + Snow X Ross's Goose Hybrid SRGH Anser caerulescens x rossii ANSCAR + Snow/Ross's Goose SRGO Anser caerulescens/rossii ANSCRO Ross's Goose -

Taxonomic List of the Birds of Utah (Jan 2021 - 467 Species)
Taxonomic List of the Birds of Utah (Jan 2021 - 467 species) Names and order according to the 58th supplement to the American Outline Structure: Ornithologists’ Union Check-list of North American Birds ORDER (-FORMES) FAMILY (-DAE) (I) = introduced species “Utah Bird Records Committee” Subfamily (-nae) www.utahbirds.org/RecCom Genus species Common Name ANSERIFORMES Mergus serrator Red-breasted Merganser ANATIDAE Oxyura jamaicensis Ruddy Duck Dendrocygninae GALLIFORMES Dendrocygna bicolor Fulvous Whistling-Duck ODONTOPHORIDAE Anserinae Callipepla squamata Scaled Quail Anser caerulescens Snow Goose Callipepla californica California Quail Anser rossii Ross's Goose Callipepla gambelii Gambel's Quail Anser albifrons Greater White-fronted Goose PHASIANIDAE Branta bernicla Brant Phasianinae Branta hutchinsii Cackling Goose Alectoris chukar Chukar (I) Branta canadensis Canada Goose Perdix perdix Gray Partridge (I) Cygnus buccinator Trumpeter Swan Phasianus colchicus Ring-necked Pheasant (I) Cygnus columbianus Tundra Swan Tetraoninae Anatinae Bonasa umbellus Ruffed Grouse Aix sponsa Wood Duck Centrocercus urophasianus Greater Sage-Grouse Spatula querquedula Garganey Centrocercus minimus Gunnison Sage- Grouse Spatula discors Blue-winged Teal Lagopus leucura White-tailed Ptarmigan Spatula cyanoptera Cinnamon Teal Dendragapus obscurus Dusky Grouse Spatula clypeata Northern Shoveler Tympanuchus phasianellus Sharp-tailed Grouse Mareca strepera Gadwall Meleagridinae Mareca penelope Eurasian Wigeon Meleagris gallopavo Wild Turkey Mareca americana American -

Use of Floral Nectar in Heliconia Stilesii Daniels by Three Species of Hermit Hummingbirds
The Condor89~779-787 0 The Cooper OrnithologicalSociety 1987 ECOLOGICAL FITTING: USE OF FLORAL NECTAR IN HELICONIA STILESII DANIELS BY THREE SPECIES OF HERMIT HUMMINGBIRDS FRANK B. GILL The Academyof Natural Sciences,Philadelphia, PA 19103 Abstract. Three speciesof hermit hummingbirds-a specialist(Eutoxeres aquikz), a gen- eralist (Phaethornissuperciliosus), and a thief (Threnetesruckerz]-visited the nectar-rich flowers of Heliconia stilesii Daniels at a lowland study site on the Osa Peninsula of Costa Rica. Unlike H. pogonanthaCufodontis, a related Caribbean lowland specieswith a less specialized flower, H. stilesii may not realize its full reproductive potential at this site, becauseit cannot retain the services of alternative pollinators such as Phaethornis.The flowers of H. stilesii appear adapted for pollination by Eutoxeres, but this hummingbird rarely visited them at this site. Lek male Phaethornisvisited the flowers frequently in late May and early June, but then abandonedthis nectar sourcein favor of other flowers offering more accessiblenectar. The strong curvature of the perianth prevents accessby Phaethornis to the main nectar chamber; instead they obtain only small amounts of nectar that leaks anteriorly into the belly of the flower. Key words: Hummingbird; pollination; mutualism;foraging; Heliconia stilesii; nectar. INTRODUCTION Ultimately affectedare the hummingbird’s choice Species that expand their distribution following of flowers and patterns of competition among speciationenter novel ecologicalassociations un- hummingbird species for nectar (Stiles 1975, related to previous evolutionary history and face 1978; Wolf et al. 1976; Feinsinger 1978; Gill the challenges of adjustment to new settings, 1978). Use of specificHeliconia flowers as sources called “ecological fitting” (Janzen 1985a). In the of nectar by particular species of hermit hum- case of mutualistic species, such as plants and mingbirds, however, varies seasonally and geo- their pollinators, new ecological settingsmay in- graphically (Stiles 1975). -
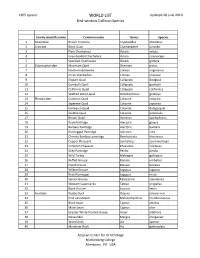
WORLD LIST Updated 28 June 2019 Bird-Window Collision Species
1305 species WORLD LIST Updated 28 June 2019 Bird-window Collision Species Family scientific name Common name Genus Species 1 Tinamidae Brown Tinamou Crypturellus obsoletus 2 Cracidae Black Guan Chamaepetes unicolor 3 Plain Chachalaca Ortalis vetula 4 Grey-headed Chachalaca Ortalis cinereiceps 5 Speckled Chachalaca Ortalis guttata 6 Odontophoridae Mountain Quail Oreortyx pictus 7 Northern Bobwhite Colinus virginianus 8 Crested Bobwhite Colinus cristatus 9 Elegant Quail Callipepla douglasii 10 Gambel's Quail Callipepla gambelii 11 California Quail Callipepla californica 12 Spotted Wood-quail Odontophorus guttatus 13 Phasianidae Common Quail Coturnix coturnix 14 Japanese Quail Coturnix japonica 15 Harlequin Quail Coturnix delegorguei 16 Stubble Quail Coturnix pectoralis 17 Brown Quail Synoicus ypsilophorus 18 Rock Partridge Alectoris graeca 19 Barbary Partridge Alectoris barbara 20 Red-legged Partridge Alectoris rufa 21 Chinese Bamboo-partridge Bambusicola thoracicus 22 Copper Pheasant Syrmaticus soemmerringii 23 Common Pheasant Phasianus colchicus 24 Grey Partridge Perdix perdix 25 Wild Turkey Meleagris gallopavo 26 Ruffed Grouse Bonasa umbellus 27 Hazel Grouse Bonasa bonasia 28 Willow Grouse Lagopus lagopus 29 Rock Ptarmigan Lagopus muta 30 Spruce Grouse Falcipennis canadensis 31 Western Capercaillie Tetrao urogallus 32 Black Grouse Lyrurus tetrix 33 Anatidae Ruddy Duck Oxyura jamaicensis 34 Pink-eared Duck Malacorhynchus membranaceus 35 Black Swan Cygnus atratus 36 Mute Swan Cygnus olor 37 Greater White-fronted Goose Anser albifrons 38 -

Hummingbird Flight Speeds
HUMMINGBIRD FLIGHT SPEEDS FRANK B. GILL TheAcademy of NaturalSciences, Philadelphia, Pennsylvania 19103 USA ABSTR^CT.--Long-tailedHermits (Phaethornissuperciliosus) normally fly fasterthan the ve- locity predictedto minimize their costof transport.The averagespeed we measuredfor individualsflying a known40-m course was 11.5 m/s. Rapidflower visitation yields rewards thatcould compensate for the extracosts of fastflight. Received 2 March 1984, accepted 30 July 1984. How fast should a bird fly? Intermediate relate to differencesin flight ecology (Fein- flight speeds that minimize instantaneous singerand Chaplin 1975,Feinsinger et al. 1979). power costs (Vmp),or more realistically that Theoretically,forward flight speedsalso should minimize the costof flying a certain distance influencea hummingbird'sflight costsand op- (Vm,),can be predicted from aerodynamicthe- timal wing lengths (Pennycuick1969, Greene- ory that is devoid of ecological context. The wait 1975);however, these speeds have not been potential for minimization of power costsat determined in an ecological context. intermediatespeeds emerges from the U-shaped Here I considerthe flight speedsof a 6-g her- form of the relationshipbetween power costs mit hummingbird, the Long-tailed Hermit and flight speed(Fig. 1; Pennycuick1969, 1975; (Phaethornissuperciliosus). Hermit humming- Greenewalt 1975). birds relate directly to the questionsposed When can birds justify the extra energetic above becauseforward flight is the dominant investment required for flight at speedsslower componentof their routine flight ecology.They or faster than Vm,?The advantagesof power fly considerabledistances between dispersed dives, top-speed chases,and escape flights flowers (Stiles 1979). Some speciesof hermit clearlyoutweigh the energeticsacrifices of brief hummingbirds,including the speciesfeatured high-speed flights. Stationary flight, such as here,also commute frequently from lek display hoveringat flowers,also may yield net rewards grounds to distant flowers. -

High Andes to Vast Amazon
Tropical Birding Trip Report EASTERN ECUADOR October-November 2016 A Tropical Birding SET DEPARTURE tour EASTERN ECUADOR: High Andes to Vast Amazon Main tour: 29th October – 12th November 2016 Tropical Birding Tour Leader: Jose Illanes This Wire-tailed Manakin was seen in the Amazon INTRODUCTION: This was always going to be a special for me to lead, as we visited the area where I was born and raised, the Amazon, and even visited the lodge there that is run by the community I am still part of today. However, this trip is far from only an Amazonian tour, as it started high in Andes (before making its way down there some days later), above the treeline at Antisana National Park, where we saw Ecuador’s national bird, the Andean Condor, in addition to Ecuadorian Hillstar, 1 www.tropicalbirding.com +1-409-515-9110 [email protected] Page Tropical Birding Trip Report EASTERN ECUADOR October-November 2016 Carunculated Caracara, Black-faced Ibis, Silvery Grebe, and Giant Hummingbird. Staying high up in the paramo grasslands that dominate above the treeline, we visited the Papallacta area, which led us to different high elevation species, like Giant Conebill, Tawny Antpitta, Many-striped Canastero, Blue-mantled Thornbill, Viridian Metaltail, Scarlet-bellied Mountain-Tanager, and Andean Tit-Spinetail. Our lodging area, Guango, was also productive, with White-capped Dipper, Torrent Duck, Buff-breasted Mountain Tanager, Slaty Brushfinch, Chestnut-crowned Antpitta, as well as hummingbirds like, Long-tailed Sylph, Tourmaline Sunangel, Glowing Puffleg, and the odd- looking Sword-billed Hummingbird. Having covered these high elevation, temperate sites, we then drove to another lodge (San Isidro) downslope in subtropical forest lower down. -

Distribution of Iridescent Colours in Hummingbird Communities Results
Distribution of iridescent colours in hummingbird communities results from the interplay between selection for camouflage and communication Hugo Gruson, Marianne Elias, Juan Parra, Christine Andraud, Serge Berthier, Claire Doutrelant, Doris Gomez To cite this version: Hugo Gruson, Marianne Elias, Juan Parra, Christine Andraud, Serge Berthier, et al.. Distribution of iridescent colours in hummingbird communities results from the interplay between selection for camouflage and communication. 2020. hal-02372238v2 HAL Id: hal-02372238 https://hal.archives-ouvertes.fr/hal-02372238v2 Preprint submitted on 3 Feb 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Copyright RESEARCH ARTICLE Open Access Distribution of iridescent colours in Open Peer-Review hummingbird communities results Open Data from the interplay between Open Code selection for camouflage and communication Cite as: Hugo G, Marianne E, Juan L. P, Christine A, Serge B, Claire D, and Doris G. Distribution of iridescent colours in hummingbird communities results from the interplay between Hugo Gruson1, Marianne Elias2, Juan L. Parra3, Christine selection for camouflage and communication. BioRxiv 586362, v5 4 5 1 peer-reviewed and recommended by PCI Andraud , Serge Berthier , Claire Doutrelant , & Doris Evolutionary Biology (2019).