A View of Healthcare in Wales Post Brexit: Preparing for the Future Welcome
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Academi Wales Annual Report 2015-16
AcademiWales: Annual Report 2015-16 AcademiWales #PublicServiceWales Annual Report 2015-16 i Contents Infographic Foreword 2 Who we work with 3 About us 4 What we do 5 Public Service Values and Behaviours 6 Conferences and schools 8 Development programmes 14 Master classes and expert seminars 20 Leadership and Organisational Development 21 Recognising excellence 24 Publications 25 Reputation and engagement 26 Mae’r ddogfen yma hefyd ar gael yn Gymraeg. This document is also available in Welsh. © Crown copyright 2016 Digital ISBN 978 1 4734 7162 7 WG27797 ii AcademiWales: Annual Report 2015-16 We trained a total of Average cost delegates per 9,383 £132 delegate 333 training days 85% delivered We delivered training We designed We received to more than and delivered positive feedback from our events and programmes 5 with the average 150 large scale being conferences organisations & scholar 97% behaviours We launched the Public Service 171,012 Values and views on our Behaviours website 2,894 Twitter 2,490 followers Bulletin subscribers Foreword Academi Wales has formed part of the portfolio experiences, graduate training schemes and of the Minister for Public Services for the past international learning opportunities. 15 months. In his very first speech leading this portfolio the Minister remarked that The ongoing guidance of our Advisory Board ‘Academi Wales has been doing good work has been crucial to our success, shaping and under the radar and it was now time to raise its supporting our strategic and operational efforts, profile… and we have. as well as acting as ambassadors for Academi Wales and our work. -

Report of Acting Chair March 2017
15.230317 Report of Acting Chair March 2017 Author: Professor Simon Smail, Acting Chair, Public Health Wales Date: 13 March 2017 Version: 1 Sponsoring Director: Professor Sir Mansel Aylward Who will present: Professor Simon Smail Date of Board / Committee meeting: 23 March 2017 Committee/Groups that have received or considered this paper: N/A The Board / Committee are asked to: Approve the recommendation(s) proposed in the paper. Discuss and scrutinise the paper and provide feedback and comments. Receive the paper for information only. X Link to Public Health Wales commitment and priorities for action: (please tick which commitment(s) is/are relevant) X X X X Priorities for action Relevant to all priorities of action Public Health Wales Acting Chair’s report – March 2017 Introduction Since 1 December 2017 Professor Simon Smail has been performing the role of Acting Chair of Public Health Wales as Sir Mansel Aylward has been suffering from ill health. Sir Mansel has commenced with his return to the organisation and given his commitment to a range of organisations/reviews, such as the Parliamentary Review he will not return fully into his role under the until 1 April 2017. This report focuses on the engagements/activities of Professor Simon Smail as the Acting Chair. Other activities performed by Sir Mansel are also shown which he has undertaken as part of a phased return to the role of Chair (see Appendix 1). Professor Simon Smail will step down as Acting Chair on the 1 April 2017 when Sir Mansel will assume the full range of roles and responsibilities. -

Seminar Report
Implementing the Wales Tobacco Control Action Plan: new research & best practice Report by the Bevan Foundation of a seminar held on July 2012, All Nations Centre, Cardiff Funded by Pfizer Implementing the Wales TCAP: seminar report Introduction The Welsh Government’s Tobacco Control Action Plan aims to reduce the prevalence of smoking amongst the Welsh population to 16% of the adult population by 2020. This target is extremely challenging and achieving it requires concerted action by everyone with a role in tobacco control. The Bevan Foundation is an influential, independent think tank which makes Wales a fairer place through research, conferences and events. The Foundation is concerned that smoking is a major factor in inequality in health between different socio- economic groups, and is anxious that the very high smoking prevalence amongst low-income groups, pregnant women and others are recognised and specifically addressed. The Bevan Foundation is pleased to have worked with Pfizer Ltd to investigate the question of smoking cessation amongst lower socio-economic groups in Wales. As part of that programme of work the Bevan Foundation worked with Pfizer Ltd to organise a seminar in July 2012 to look at good practice in reaching disadvantaged groups of smokers. The programme included examples from Wales and England, which provided a unique insight into the range of different, effective interventions that can be made. The response of delegates was extremely positive. In order to reach as wide an audience as possible, we have therefore produced this brief summary of the seminar. A copy of the seminar programme is in Annex 1 and biographies of the speakers are at Annex 2. -

Location Guide Accessible from All Parts of Britain
Routes to Cardiff Cardiff University By Road (£3.00 per person). See Map 2 for “One of the top teaching and experience we believe should be open Cardiff is served by the M4 and is easily location. Car parking in the University research institutions in the UK. to all, encouraging students from the Location Guide accessible from all parts of Britain. From car parks is extremely limited and a It has excellent facilities, often most deprived areas to apply and permit is required. However, there are succeed on Cardiff courses. the south west, take the M5 and from magnificent buildings, great several public car parks located close to When they arrive in Cardiff, our the south of England, follow major social and sporting facilities, 2013 A-roads to the M4. From Scotland, the University, all of which are marked students benefit from a stimulating the north of England and the Midlands, on Map 4. There is also pay and display wrapped up in a vibrant, cultural study environment, research-led travel via the M50 to the M4. car parking available on Park Place and city centre.” teaching and interaction with within the civic centre (along College academics working at the frontiers of Travelling east on the M4 . Leave the Road, City Hall Road, King Edward VII Sunday Times Good University Guide knowledge in their field. motorway at Junction 32, follow the Avenue and Museum Avenue). This Cardiff University is about the freedom As a major higher education provider for A470, sign-posted City Centre. typically costs around £3.50 for two to explore, to learn and to discover. -
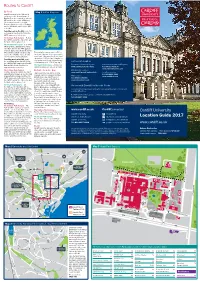
Location Guide 2017
Routes to Cardiff By Road Map 1 United Kingdom Cardiff is served by the M4 and is easily accessible from all parts of Britain. From the south west, take the M5 and from the south of England, follow major A-roads to the M4. From Scotland, the north of England and the Midlands, travel via the M50 to the M4. Travelling east on the M4. Leave the motorway at Junction 32, follow the A470, sign-posted city centre. The Cathays Park Campus – Follow the A470 towards the city centre and the University is sign-posted. The Heath Park Campus – Follow the A470 as far as Gabalfa Interchange. Take first exit onto the A48 (Eastern Avenue), sign-posted Newport and the M4, but immediately exit at the slip This typically costs around £3.50 for road on the left. This leads directly two hours. Payment is by coin or debit/ onto the University Hospital site. credit card. Parking in the North Road car park is £5.00 per day. (Charges for Travelling west on the M4. Leave city centre multi-storey car parks vary the motorway at Junction 29, follow considerably, up to £18.00 per day.) Further Information the A48(M)/A48, sign-posted Cardiff Door-to-door directions by road Coach services (National Express) East and the South. Continue for The Heath Park Campus – Limited car approximately seven miles. parking is available in various car parks. www.cardiff.ac.uk/directions Tel: (08717) 818178 www.nationalexpress.com The Cathays Park Campus – By Rail / Coach / Bus Park and Ride services Continue along the A48 to the A470 High speed inter-city trains provide www.cardiff.gov.uk/parkandride Local bus services (Gabalfa Interchange). -

Regional Senedd Situation of Polling Stations Eng
Senedd Regional Election: South Wales Central Region Situation of Polling Stations Etholiad Rhanbarthol Senedd: Rhanbarth Canol De Cymru Lleoliad Gorsafoedd Pleidleisio No of DESCRIPTION OF No of DESCRIPTION OF No of DESCRIPTION OF Polling SITUATION OF POLLING STATION PERSONS Polling SITUATION OF POLLING STATION PERSONS Polling SITUATION OF POLLING STATION PERSONS Station ENTITLED TO VOTE Station ENTITLED TO Station ENTITLED TO VOTE VOTE Nifer y LLEOLIAD YR ORSAF BLEIDLEISIO DISGRIFIAD O’R Nifer y LLEOLIAD YR ORSAF BLEIDLEISIO Nifer y LLEOLIAD YR ORSAF BLEIDLEISIO gorsafoedd RHAI A GAIFF gorsafoedd DISGRIFIAD O’R gorsafoed DISGRIFIAD O’R pleidleisio BLEIDLEISIO pleidleisio RHAI A GAIFF d RHAI A GAIFF BLEIDLEISIO pleidleisio BLEIDLEISIO The poll at the Senedd election is to be taken together with the poll at the Police and Crime Commissioner election for the South Wales Police Area. Mae'r bleidlais yn etholiad y Senedd i'w chynnal ynghyd â'r bleidlais yn etholiad Comisiynydd yr Heddlu a Throsedd ar gyfer Ardal Heddlu De Cymru. PORTACABIN - NEAR HOME FARM, OE0/OE HOLY TRINITY CHURCH UF0/UG 1 ST FRANCIS MILLENNIUM CENTRE AA0 37 MICHAELSTON LE PIT 73 1/OF0 0/UH0 PORTACABIN - CAR PARK, BRON Y WENVOE COMMUNITY CENTRE PA0/PA ST ATHAN COMMUNITY CENTRE VA0/VC 2 MOR, BARRY AB0 38 74 1/PB0 0 ST NICHOLAS HALL ST NICHOLAS CHURCH RAF ST ATHAN GOLF CLUB 3 AB1 39 PC0 75 VB0 BARRY ISLAND COMMUNITY BONVILSTON READING ROOM ALL SAINTS CHURCH WA0/W 4 CENTRE AC0 40 PD0 76 B0 PORTACABIN PREMIER INN ST GEORGES VILLAGE HALL QA0/QB ST BRIDES MAJOR CHURCH HALL -

NHS Wales Shared Services Partnership Have Delivered on Behalf of Health Boards and NHS Trusts Across Wales
IN Adding Value through Partnership PARTNERSHIP Magazine Summer edition, 2016 Procurement Support New Resource Centre In Partnership Summer Edition 2016 2 Contents Page Success for NWSSP TEL Team at Prestigous HPMA 04 Awards Ceremony Daniela Becomes Welsh Representative for Employment 05 Lawyers Association 06 NWSSP Colleague Leads the Way at British Dietetic Association Welsh Board Conference WEDS Support Development of Medical Workforce 07 Strategy for NHS Wales NWSSP Colleagues Take the Welsh 3 Peaks Challenge for 08 Velindre Cancer Centre Primary Care Services Director Nominated as Finalist in 10 Institute of Directors (IoD) Wales Awards 11 Primary Care Services Re-attain Customer Service Excellence Award Procurement Support New Resource Centre for Welsh Ambulance Service 12 & North Wales Fire & Rescue Service Procurement Work With Public Health Wales in Innovative 14 ‘Our Space’ Evaluation Procurement Services Support Welsh Blood Service 15 Through Expansion Process Standardisation at Hywel Dda University Health Board 16 with Implementation of New ‘Pumps Project’ 17 Primary Care Services on the Road An Update on the Implementation of a Centralised System for Claiming 18 Relocation Expenses for Doctors & Dentists Training Grades Collaborative Commissioning Programme Inaugural Project on 20 Care Homes Appliances and Dressings Sourcing Team Winners at National Patient 22 Safety Awards NWSSP Colleagues Honoured as part of First Unison 23 Health Awards 24 ISO9001 and OHSAS18001 Audit Success for Procurement Services Fraud Doctor is Told to Repay £75,000 or Face Two Years in Jail After He 25 Moonlighted While Claiming Sick Pay 26 Counter Fraud: Help Protect your NHS from Fraud 28 Engaging with Health Boards & Trusts at the All Wales Planning Event In Partnership Summer Edition 2016 3 Welcome to “In Partnership”, the Magazine for Our Staff, Health Boards & Trusts This publication aims to highlight some of the recent achievements that the NHS Wales Shared Services Partnership have delivered on behalf of Health Boards and NHS Trusts across Wales. -

Enclosure No: 1/AWMSG/0220 Agenda Item No: 1 – Minutes of Previous Meeting Author: Chair, AWMSG Tel: 029 20716900 Contact: E-Mail: [email protected]
Enclosure No: 1/AWMSG/0220 Agenda Item No: 1 – Minutes of previous meeting Author: Chair, AWMSG Tel: 029 20716900 Contact: E-Mail: [email protected] ALL WALES MEDICINES STRATEGY GROUP (AWMSG) Minutes of the AWMSG meeting held Wednesday, 11th December 2019 commencing 10.30 am at the Copthorne Hotel, Copthorne Way Culverhouse Cross, Cardiff, CF5 6DH Did not VOTING MEMBERS PRESENT: participate in 1. Prof Ceri Phillips Chair 2. Prof Iolo Doull WHSSC 3. Prof Stephen Monaghan Consultant in Public Health Medicine 4. Dr Jeremy Black General Practitioner 5. Mr Hywel Pullen Director of Finance 6. Mr Cliff Jones Lay Member 7. Mrs Alison Hughes Primary Care Pharmacist 8. Mr Tommy Price ABPI Cymru Wales 9. Prof Dyfrig Hughes Health Economist 10. Mrs Louise Williams Senior Nurse 11. Mr Stefan Fec Community Pharmacist 12. Mr John Terry Managed Sector Secondary Care Pharmacist AWTTC staff in attendance: Dr James Coulson, Interim Clinical Director & NMG Chai Mrs Ruth Lang, Senior Liaison Manager Ms Kath Haines, Head of WAPSU Mr Tony Williams, Senior Appraisal Pharmacist - Team Leader Mr Richard Boldero, Pharmacist Dr Stephanie Francis, Senior Scientist Dr Stuart Keeping, Senior Scientist Page 1 of 8 AWMSG draft minutes December 2019 Prepared by AWTTC List of Abbreviations: ABPI Association of the British Pharmaceutical Industry ASAR AWMSG Secretariat Assessment Report AWMSG All Wales Medicines Strategy Group AWPAG All Wales Prescribing Advisory Group AWTTC All Wales Therapeutics & Toxicology Centre BMA British Medical Association CAPIG Clinical and Patient -

CYFARFOD BWRDD PRIFYSGOL IECHYD UNIVERSITY HEALTH BOARD MEETING DYDDIAD Y CYFARFOD: DATE of MEETING: 28 September 2017 TEITL YR
CYFARFOD BWRDD PRIFYSGOL IECHYD UNIVERSITY HEALTH BOARD MEETING DYDDIAD Y CYFARFOD: 28 September 2017 DATE OF MEETING: TEITL YR ADRODDIAD: Board Development Programme TITLE OF REPORT: CYFARWYDDWR ARWEINIOL: Bernardine Rees, OBE, Chair LEAD DIRECTOR: Steve Moore, Chief Executive SWYDDOG ADRODD: Joanne Wilson, Board Secretary REPORTING OFFICER: Christine Davies, Head of Organisational Development Pwrpas yr Adroddiad (dewiswch fel yn addas) Purpose of the Report (select as appropriate) Ar Gyfer Penderfyniad/For Decision ADRODDIAD SCAA SBAR REPORT Sefyllfa / Situation This paper details the proposed approach to and content of, Hywel Dda University Health Board’s Board Development Programme. The proposal, building on work previously undertaken, has been developed in order to provide ongoing support to the Board, focusing on key development areas that – once completed – will provide Members with enhanced knowledge, skills and behaviours, thereby improving individual and collective performance. Cefndir / Background The Welsh Government is placing increasing focus on driving improvement in the economy and delivery of public services. The Board Members of public services organisations have a key role in ensuring that the policies and priorities of the Welsh Government are implemented and delivered. Enabling personal development and learning, building capability and capacity in individuals and organisations, is therefore key to successful delivery of an organisation’s aims and objectives. A local Board Development Programme, tailored to meet the needs of the organisation and both its Independent and Executive Director Board Members is therefore paramount in pursuing the above objective. Asesiad / Assessment Further to previous Board Development work undertaken, including the elements delivered by Academi Wales, a steering group was formed in order to build on the work already completed. -

Wales. Newsletter
Wales. Newsletter Summer 2019 Edition Associate Editor Dr Anita Naik RCPsych Wales, Policy & Public Affairs Attachment rcpsych.ac.uk/wales CAMHS Higher Trainee @RCPsychWales 1 | welcome Professor Keith Lloyd Chair, RCPsych Wales Vice President, Royal College of Psychiatrists Welcome to the summer edition of the Royal College of Psychiatrists Wales newsletter. I can’t quite believe we’ve reached July already but here we are! The first edition of the newsletter proved to be a tremendous success, so thanks to all of you who provided feedback and offered suggestions regarding content for this one. A special thanks to Dr Anita Naik for taking on the role of associate editor for this edition – we’re really grateful, and her article is an important read. It’s been another incredibly busy time again for us all here at the college, and things show no sign of slowing down! In these past few months, we’ve been responding to numerous government consultations, hosted more exciting and innovative events, achieved increasing media coverage and requests for our input into features and stories. In this edition you’ll find contributions from our members, partner organisations and overall plenty of news and information about the work we’re all doing here in Wales. therapies in secondary care for which eligibility criteria are used to manage demand. On a personal note, there have a been a few issues I’ve been watching closely of late, and they Finally as you can see from the content of this continue to be consistent themes for the college newsletter, there are many positive things to to focus upon: celebrate too such as the 100% fill rate for general psychiatry core training this year. -

Email 1 Lynne Kelly
Email 1 From: [email protected] <[email protected]> Sent: 20 July 2018 09:54 To: Redacted – s40 (2) (HSS-DPH-Population Healthcare) <@gov.wales> Subject: Core Participants Hi Redacted – s40 (2) Thomas Powell was the Inquiry solicitor who came to Cardiff with Catherine Nallty. I think he will lead for Wales, he's Australian (I think) and has connections with Abergavenny. The Inquiry are asking for expressions of interest to be made by today, 20th July for both witnesses and core participants His email address Redacted – s40 (2) @infectedbloodinquiry.org.uk Regards Lynne Kelly Chair of Haemophilia Wales www.haemophiliawales.org [email protected] Registered Charity No: 1158941 Email 2 From: [email protected] <[email protected]> Sent: 26 February 2018 09:11 To: Redacted – s40 (2) (HSS-DPH-Population Healthcare) <@gov.wales> Subject: Public Inquiry Contaminated Blood Hi Redacted – s40 (2) I hope you are well. I have had a call from the Deputy Secretary to the Inquiry Catherine McNulty. They are planning some consultation meetings with Justice Brian Langstaff, Chair of the Inquiry and the various groups. Would it be possible for myself and Michael Imperato to meet with you to discuss further? Many Thanks Lynne Lynne Kelly Chair of Haemophilia Wales www.haemophiliawales.org [email protected] Registered Charity No: 1158941 Email 3 From: [email protected] <[email protected]> Sent: 03 March 2018 10:56 To: Redacted – s40 (2) (HSS-DPH-Population Healthcare) <@gov.wales>; Redacted – s40 (2) (HSS- DPH-Population Healthcare) <@gov.wales> Subject: FYI Consultation on Terms of Reference Dear Redacted – s40 (2) and Redacted – s40 (2) I wanted to let you know that Haemophilia Wales and Michael Imperato our legal representative will be meeting Justice Langstaff on 14th March to discuss Terms of Reference for the Inquiry. -

All Wales National Thrombosis Conference 2020
Awarded 5 CPD credits All Wales National Thrombosis Conference 2020 Wednesday 21st October 2020 The All Nations Centre, Sachville Avenue, Heath, Cardiff, CF14 3NY 08:15 – 09:00 - Registration - 09:00 – 09:15 Introduction Morning Chair –Professor Simon Noble Cancer Associated Thrombosis: Professor Simon Noble, Clinical Professor and Honorary Consultant 09:15 – 10:00 Latest Updates in management in Palliative Medicine Updates on NICE guidance including Dr Heledd Roberts DOACs and anti phospholipid Consultant Haematologist syndrome University Hospital Wales, Cardiff 10:00 – 10:30 Compression and VTE – how does it Dr Rhys Morris work? Clinical Scientist University Hospital Wales, Cardiff 10:30 – 11:00 Coffee and Exhibitor Break Key-note Speaker (am) Professor Roopen Arya Professor of Thrombosis and haemostasis 11:00 – 11:30 Where next with VTE prevention? Kings College Hospital NHS Foundation 11:30 – 12:00 Implementation of VTE prophylaxis in Dr Nagappan Kumar surgery Lead Consultant Hepato-biliary Surgery University Hospital Wales, Cardiff 12:00 – 12:10 Q & A Morning Speakers 12:10 – 13:10 Lunch and Exhibitor Break Afternoon Chair: Andrea Croft Workshop 1 Workshop 2 Workshop 3 35 mins session 35 mins session 35 mins session Complex VTE Case Studies DOAC Management Exploring New Anticoagulant Therapies Case Studies Dr Heledd Roberts, Consultant Dr Samya Obaji, Tristan Groves Haematologist Consultant Haematologist Pharmacist C & V UHB C & V UHB C & V UHB S1. 13:10 –13:45 S1. 13:10 – 13:45 S1. 13:10 – 13:45 S2. 13:45 -14:20 S2. 13:45 - 14:20 S2. 13:45 - 14:20 S3. 14:20 –14:55 S3.