Basic Science of Osteoarthritis Magali Cucchiarini1*, Laura De Girolamo2, Giuseppe Filardo3, J
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pg 131 Chondroblast -> Chondrocyte (Lacunae) Firm Ground Substance
Figure 4.8g Connective tissues. Chondroblast ‐> Chondrocyte (Lacunae) Firm ground substance (chondroitin sulfate and water) Collagenous and elastic fibers (g) Cartilage: hyaline No BV or nerves Description: Amorphous but firm Perichondrium (dense irregular) matrix; collagen fibers form an imperceptible network; chondroblasts produce the matrix and when mature (chondrocytes) lie in lacunae. Function: Supports and reinforces; has resilient cushioning properties; resists compressive stress. Location: Forms most of the embryonic skeleton; covers the ends Chondrocyte of long bones in joint cavities; forms in lacuna costal cartilages of the ribs; cartilages of the nose, trachea, and larynx. Matrix Costal Photomicrograph: Hyaline cartilage from the cartilages trachea (750x). Thickness? Metabolism? Copyright © 2010 Pearson Education, Inc. Pg 131 Figure 6.1 The bones and cartilages of the human skeleton. Epiglottis Support Thyroid Larynx Smooth Cartilage in Cartilages in cartilage external ear nose surface Cricoid Trachea Articular Lung Cushions cartilage Cartilage of a joint Cartilage in Costal Intervertebral cartilage disc Respiratory tube cartilages in neck and thorax Pubic Bones of skeleton symphysis Meniscus (padlike Axial skeleton cartilage in Appendicular skeleton knee joint) Cartilages Articular cartilage of a joint Hyaline cartilages Elastic cartilages Fibrocartilages Pg 174 Copyright © 2010 Pearson Education, Inc. Figure 4.8g Connective tissues. (g) Cartilage: hyaline Description: Amorphous but firm matrix; collagen fibers form an imperceptible network; chondroblasts produce the matrix and when mature (chondrocytes) lie in lacunae. Function: Supports and reinforces; has resilient cushioning properties; resists compressive stress. Location: Forms most of the embryonic skeleton; covers the ends Chondrocyte of long bones in joint cavities; forms in lacuna costal cartilages of the ribs; cartilages of the nose, trachea, and larynx. -
Introduction
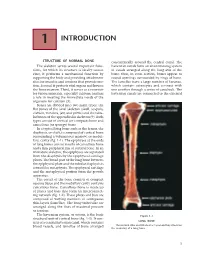
1 INTRODUCTION STRUCTURE OF NORMAL BONE concentrically around the central canal. The The skeleton serves several important func- haversian canals form an anastomosing system tions, for which its structure is ideally suited. of canals arranged along the long axis of the First, it performs a mechanical function by bone; thus, in cross section, bones appear as supporting the body and providing attachment round openings surrounded by rings of bone. sites for muscles and tendons that provide mo- The lamellae have a large number of lacunae, tion. Second, it protects vital organs and houses which contain osteocytes and connect with the bone marrow. Third, it serves as a reservoir one another through a series of canaliculi. The for various minerals, especially calcium, and has haversian canals are connected to the external a role in meeting the immediate needs of the organism for calcium (3). Bones are divided into two main types: the flat bones of the axial skeleton (skull, scapula, clavicle, vertebra, jaw, and pelvis) and the tubu- lar bones of the appendicular skeleton (9). Both types consist of cortical (or compact) bone and cancellous (or spongy) bone. In a typical long bone such as the femur, the diaphysis, or shaft, is composed of cortical bone surrounding a voluminous marrow, or medul- lary, cavity (fig. 1-1). The epiphyses at the ends of long bones consist mostly of cancellous bone and a thin peripheral rim of cortical bone. In an immature skeleton, the epiphyses are separated from the diaphysis by the epiphyseal cartilage plates. The broad part of the long bone between the epiphyseal plate and the tubular diaphysis is termed the metaphysis. -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

Biology of Bone Repair
Biology of Bone Repair J. Scott Broderick, MD Original Author: Timothy McHenry, MD; March 2004 New Author: J. Scott Broderick, MD; Revised November 2005 Types of Bone • Lamellar Bone – Collagen fibers arranged in parallel layers – Normal adult bone • Woven Bone (non-lamellar) – Randomly oriented collagen fibers – In adults, seen at sites of fracture healing, tendon or ligament attachment and in pathological conditions Lamellar Bone • Cortical bone – Comprised of osteons (Haversian systems) – Osteons communicate with medullary cavity by Volkmann’s canals Picture courtesy Gwen Childs, PhD. Haversian System osteocyte osteon Picture courtesy Gwen Childs, PhD. Haversian Volkmann’s canal canal Lamellar Bone • Cancellous bone (trabecular or spongy bone) – Bony struts (trabeculae) that are oriented in direction of the greatest stress Woven Bone • Coarse with random orientation • Weaker than lamellar bone • Normally remodeled to lamellar bone Figure from Rockwood and Green’s: Fractures in Adults, 4th ed Bone Composition • Cells – Osteocytes – Osteoblasts – Osteoclasts • Extracellular Matrix – Organic (35%) • Collagen (type I) 90% • Osteocalcin, osteonectin, proteoglycans, glycosaminoglycans, lipids (ground substance) – Inorganic (65%) • Primarily hydroxyapatite Ca5(PO4)3(OH)2 Osteoblasts • Derived from mesenchymal stem cells • Line the surface of the bone and produce osteoid • Immediate precursor is fibroblast-like Picture courtesy Gwen Childs, PhD. preosteoblasts Osteocytes • Osteoblasts surrounded by bone matrix – trapped in lacunae • Function -

Bone Cartilage Dense Fibrous CT (Tendons & Nonelastic Ligaments) Dense Elastic CT (Elastic Ligaments)
Chapter 6 Content Review Questions 1-8 1. The skeletal system consists of what connective tissues? Bone Cartilage Dense fibrous CT (tendons & nonelastic ligaments) Dense elastic CT (elastic ligaments) List the functions of these tissues. Bone: supports the body, protects internal organs, provides levers on which muscles act, store minerals, and produce blood cells. Cartilage provides a model for bone formation and growth, provides a smooth cushion between adjacent bones, and provides firm, flexible support. Tendons attach muscles to bones and ligaments attach bone to bone. 2. Name the major types of fibers and molecules found in the extracellular matrix of the skeletal system. Collagen Proteoglycans Hydroxyapatite Water Minerals How do they contribute to the functions of tendons, ligaments, cartilage and bones? The collagen fibers of tendons and ligaments make these structures very tough, like ropes or cables. Collagen makes cartilage tough, whereas the water-filled proteoglycans make it smooth and resistant. As a result, cartilage is relatively rigid, but springs back to its original shape if it is bent or slightly compressed, and it is an excellent shock absorber. The extracellular matrix of bone contains collagen and minerals, including calcium and phosphate. Collagen is a tough, ropelike protein, which lends flexible strength to the bone. The mineral component gives the bone compression (weight-bearing) strength. Most of the mineral in the bone is in the form of hydroxyapatite. 3. Define the terms diaphysis, epiphysis, epiphyseal plate, medullary cavity, articular cartilage, periosteum, and endosteum. Diaphysis – the central shaft of a long bone. Epiphysis – the ends of a long bone. Epiphyseal plate – the site of growth in bone length, found between each epiphysis and diaphysis of a long bone and composed of cartilage. -

Calcium Salts Provide Vital Minerals -Lipids Are in Stored Yellow Marrow
10/12/2011 Primary Functions of Skeletal System 1. support 2. storage of minerals & lipids -calcium salts provide vital minerals -lipids are in stored yellow marrow 3. blood cell production -RBC’s, WBC’s, and other constituents produced 4. protection ribs: heart & lungs skull: brain vertebrae: spinal chord etc. 5. leverage: w/o bone contracting muscles just get short & fat Classification of Bones 3. Flat bones (thin roughly parallel surfaces) • Every adult skeleton contains 206 bones which can be arranged into six broad categories • Roof of skull, ribs, the sternum, scapula according to shape • Provide great protection 1. Long bones (relatively long and slender) • Provide lots of surface area for muscle • Found in arm, forearm, leg, palm, soles, attachment fingers, toes 4. Irregular bones (complex shapes with short, • The femur is the largest and heaviest bone in flat, notched or ridged surfaces) the body • Spinal vertebrae and several skull bones 2. Short Bones (box-like) • Carpal bones in wrist, tarsal bones in ankle 5. Sesamoid bones (generally small, flat, and shaped like sesame seed) • Develop inside tendons, commonly near joints i.e.- the patellae 6. Sutural bones (wormian bones) • Small, flat, irregularly shaped bones, form between flat bones of skull 1 10/12/2011 Each bone contains two types of Osseous Long bones (bone) tissue •Spaces in the joints are filled with synovial fluid 1. Compact (dense) tissue: •The epiphysis in the joint is covered by a layer of •Tightly packed hyaline cartilage called articular cartilage. •Occurs on outside surface of bone for strength •The me du llary cav ity an d the open spaces o f the & protection epiphysis are filled with marrow: 2. -

Dimensional Reconstruction of Haversian Systems in Human
Journal of Anatomy J. Anat. (2016) doi: 10.1111/joa.12430 Three-dimensional reconstruction of Haversian systems in human cortical bone using synchrotron radiation-based micro-CT: morphology and quantification of branching and transverse connections across age Isabel S. Maggiano,1,2 Corey M. Maggiano,2,3 John G. Clement,4 C. David L. Thomas,4 Yasmin Carter5 and David M. L. Cooper1 1Department of Anatomy and Cell Biology, University of Saskatchewan, Saskatoon, SK, Canada 2Department of Anthropology, University of West Georgia, Carrollton, GA, USA 3Department of Anthropology, University of Western Ontario, London, ON, Canada 4Melbourne Dental School, University of Melbourne, Melbourne, Vic., Australia 5Department of Radiology, University of Massachusetts Medical School, Worchester, MA, USA Abstract This study uses synchrotron radiation-based micro-computed tomography (CT) scans to reconstruct three- dimensional networks of Haversian systems in human cortical bone in order to observe and analyse interconnectivity of Haversian systems and the development of total Haversian networks across different ages. A better knowledge of how Haversian systems interact with each other is essential to improve understanding of remodeling mechanisms and bone maintenance; however, previous methodological approaches (e.g. serial sections) did not reveal enough detail to follow the specific morphology of Haversian branching, for example. Accordingly, the aim of the present study was to identify the morphological diversity of branching patterns and transverse connections, and to understand how they change with age. Two types of branching morphologies were identified: lateral branching, resulting in small osteon branches bifurcating off of larger Haversian canals; and dichotomous branching, the formation of two new osteonal branches from one. -

Bone and Bone Tissue Skeletal System = , , Bones Are Main Organs
61 Chapter 6: Bone and Bone Tissue Skeletal system = , , Bones are main organs: - osseous tissue - dense regular and irregular CT, plus bone marrow Module 6.1: Introduction to Bones as Organs FunctionsFUNCTIONS of OF the THE Skeletal SKELETAL SYSTEM System • Functions: 1. Protection 2. Mineral storage and 3. Blood cell formation: involved in formation of blood cells (hematopoiesis or hemopoiesis) 4. Fat storage: in yellow bone marrow of 5. Movement: bones are sites for skeletal muscle attachment 6. Support: supports weight and provides BONE STRUCTURE CLASSIFICATION (based on shape) 1. Long bones - longer than they are wide; - include most bones in arms and legs 2. Short bones – roughly cube-shaped - include carpals and 62 3. Flat bones – thin and broad bones - ribs, pelvis, sternum and 4. Irregular bones – include and certain skull bones 5. Sesamoid bones – located within BONE STRUCTURE Structure of long bone: • Periosteum – membrane surrounds outer surface • Perforating fibers (Sharpey’s fibers) - anchors periosteum firmly to bone surface • Diaphysis – • Epiphysis - of long bone (proximal & distal) • Articular cartilage – hyaline cartilage • Marrow cavity – contains bone marrow (red or yellow) • Endosteum – thin membrane lining marrow cavity Compact bone - hard, dense outer region - allows bone to resist stresses (compression & twisting) • Spongy bone ( bone) - found inside cortical bone - honeycomb-like framework of bony struts; - resist forces from many directions • EpiphySeal lines – separates epiphyses from diaphysis - remnants of -

Bone Development Histology Fundamentals > Musculoskeletal System > Musculoskeletal System
Bone Development Histology Fundamentals > Musculoskeletal System > Musculoskeletal System BONE DEVELOPMENT: OSTEOGENESIS (OSSIFICATION)  Endochondral ossification • An INDIRECT form of ossification, wherein a hyaline cartilaginous model (template) is replaced with bone, such as occurs with long bones (eg, the femur). Intramembranous ossification • A DIRECT form of ossification mesenchymal cells directly differentiate to osteoblasts (no cartilaginous model is first formed), such as occurs with flat bones (the skull bones). INTRAMEMBRANOUS OSSIFICATION  • Embryologically, skeletal tissues typically derive from mesoderm: the midline (axial) skeleton derives from the somites and the appendicular (the limb) skeleton derives from the lateral plate. • Mesenchymal cells migrate to vascularized gelatinous extracellular collagen fiber matrix (a primary spongiosa). • They differentiate directly into osteoblasts. • Osteoblasts form bone in a loosely arranged (disorganized), immature initial form of bone, called woven bone. • Osteoblasts become trapped within their own bony matrix and become osteocytes; these bony matrices are referred to as trabeculae (aka fused spicules). • Woven bone later matures to form lamellar bone, a much tougher form of bone that constitutes both compact bone and spongy bone. Compact Bone • The outer layer: the periosteum. - Comprises columns of compact bone, called osteons. - Centrally, within each osteon, lies a longitudinally-oriented canal, the Haversian (aka central) canal. - Each osteon comprises concentric rings of lamellae. - Osteocytes are a mature form of osteoblast (the bone-producing cells) within the bony matrix. - Internal to the compact bone, lies the endosteum, which comprises in inner circumferential lamellae and the 1 / 7 osteoprogenitor cells, internal to it. Spongy bone* Lies internal to the endosteum and comprises a network of lamellae that do NOT form the Haversian channels and osteons found in compact bone. -

26 April 2010 TE Prepublication Page 1 Nomina Generalia General Terms
26 April 2010 TE PrePublication Page 1 Nomina generalia General terms E1.0.0.0.0.0.1 Modus reproductionis Reproductive mode E1.0.0.0.0.0.2 Reproductio sexualis Sexual reproduction E1.0.0.0.0.0.3 Viviparitas Viviparity E1.0.0.0.0.0.4 Heterogamia Heterogamy E1.0.0.0.0.0.5 Endogamia Endogamy E1.0.0.0.0.0.6 Sequentia reproductionis Reproductive sequence E1.0.0.0.0.0.7 Ovulatio Ovulation E1.0.0.0.0.0.8 Erectio Erection E1.0.0.0.0.0.9 Coitus Coitus; Sexual intercourse E1.0.0.0.0.0.10 Ejaculatio1 Ejaculation E1.0.0.0.0.0.11 Emissio Emission E1.0.0.0.0.0.12 Ejaculatio vera Ejaculation proper E1.0.0.0.0.0.13 Semen Semen; Ejaculate E1.0.0.0.0.0.14 Inseminatio Insemination E1.0.0.0.0.0.15 Fertilisatio Fertilization E1.0.0.0.0.0.16 Fecundatio Fecundation; Impregnation E1.0.0.0.0.0.17 Superfecundatio Superfecundation E1.0.0.0.0.0.18 Superimpregnatio Superimpregnation E1.0.0.0.0.0.19 Superfetatio Superfetation E1.0.0.0.0.0.20 Ontogenesis Ontogeny E1.0.0.0.0.0.21 Ontogenesis praenatalis Prenatal ontogeny E1.0.0.0.0.0.22 Tempus praenatale; Tempus gestationis Prenatal period; Gestation period E1.0.0.0.0.0.23 Vita praenatalis Prenatal life E1.0.0.0.0.0.24 Vita intrauterina Intra-uterine life E1.0.0.0.0.0.25 Embryogenesis2 Embryogenesis; Embryogeny E1.0.0.0.0.0.26 Fetogenesis3 Fetogenesis E1.0.0.0.0.0.27 Tempus natale Birth period E1.0.0.0.0.0.28 Ontogenesis postnatalis Postnatal ontogeny E1.0.0.0.0.0.29 Vita postnatalis Postnatal life E1.0.1.0.0.0.1 Mensurae embryonicae et fetales4 Embryonic and fetal measurements E1.0.1.0.0.0.2 Aetas a fecundatione5 Fertilization -

Osteon Mimetic Scaffolding Janay Clytus University of South Carolina - Columbia, [email protected]
University of South Carolina Scholar Commons Senior Theses Honors College Spring 2018 Osteon Mimetic Scaffolding Janay Clytus University of South Carolina - Columbia, [email protected] Follow this and additional works at: https://scholarcommons.sc.edu/senior_theses Part of the Biomaterials Commons, and the Other Life Sciences Commons Recommended Citation Clytus, Janay, "Osteon Mimetic Scaffolding" (2018). Senior Theses. 252. https://scholarcommons.sc.edu/senior_theses/252 This Thesis is brought to you by the Honors College at Scholar Commons. It has been accepted for inclusion in Senior Theses by an authorized administrator of Scholar Commons. For more information, please contact [email protected]. Clytus 1 Table of Contents Thesis Summary -------------------------------------------------------------------------------------------- 2 Abstract ------------------------------------------------------------------------------------------------------ 2 Introduction ------------------------------------------------------------------------------------------------- 3 Developmental Processes that Govern Recovery ------------------------------------------------------ 6 Osteogenesis --------------------------------------------------------------------------------------- 6 Chondrogenesis ------------------------------------------------------------------------------------7 Vasculogenesis ------------------------------------------------------------------------------------ 8 Techniques to develop in-vitro vasculature ------------------------------------------------------------ -

BONE TISSUE Osteoblasts
Department of Histology and Embryology, P. J. Šafárik University, Medical Faculty, Košice BONE TISSUE: Sylabus for foreign students Author: MVDr. Zuzana Jonecová, CSc. Revised by: prof. MUDr. Eva Mechírová, CSc. BONE TISSUE - structural component of bones - specialized connective tissue for support and protection - composition: - cells - mineralized intercellular matrix – bone matrix Histological composition of the bone tissue: 1. Bone matrix – intercellular substance a. Inorganic components → Ca, P, Mg, K, Na → hyproxyapatit crystals (65%) crystals lie alongside collagen fibrills and are surrounded by amorphous ground substance b. Organic components: Fibers : collagen fibers (collagen type I ) ! Amorphous ground substance : noncollagenous proteins GAG, PG, structural GP small regulatory proteins 2. Cells a. Osteoprogenitor cells (preosteoblasts) b. Osteoblasts (bone forming cells) c. Osteocytes (inactive osteoblast) d. Osteoclasts (bone resorbing cells) Osteoblasts – line endosteal and periosteal surfaces ( 1 layer) - cuboidal cells with basophilic cytoplasm (proteosynthesis) - deposit of unmineralized organic matrix –osteoid – produced at the surface of the bone tissue when new bone tissue is required FUNCTION : 1) synthesis of organic components of bone matrix (growth, response to fracture, remodelling) 2) regulate mineralization of bone matrix 3) regulate activity of bone resorption (osteoclasts) Produce : - collagen type I (95 %) - non-collagenous bone matrix proteins : GAG, proteoglycans (hyaluronan, chondroitin and keratan sulfate)