Dimensional Reconstruction of Haversian Systems in Human
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Introduction
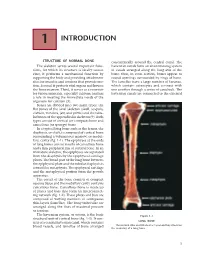
1 INTRODUCTION STRUCTURE OF NORMAL BONE concentrically around the central canal. The The skeleton serves several important func- haversian canals form an anastomosing system tions, for which its structure is ideally suited. of canals arranged along the long axis of the First, it performs a mechanical function by bone; thus, in cross section, bones appear as supporting the body and providing attachment round openings surrounded by rings of bone. sites for muscles and tendons that provide mo- The lamellae have a large number of lacunae, tion. Second, it protects vital organs and houses which contain osteocytes and connect with the bone marrow. Third, it serves as a reservoir one another through a series of canaliculi. The for various minerals, especially calcium, and has haversian canals are connected to the external a role in meeting the immediate needs of the organism for calcium (3). Bones are divided into two main types: the flat bones of the axial skeleton (skull, scapula, clavicle, vertebra, jaw, and pelvis) and the tubu- lar bones of the appendicular skeleton (9). Both types consist of cortical (or compact) bone and cancellous (or spongy) bone. In a typical long bone such as the femur, the diaphysis, or shaft, is composed of cortical bone surrounding a voluminous marrow, or medul- lary, cavity (fig. 1-1). The epiphyses at the ends of long bones consist mostly of cancellous bone and a thin peripheral rim of cortical bone. In an immature skeleton, the epiphyses are separated from the diaphysis by the epiphyseal cartilage plates. The broad part of the long bone between the epiphyseal plate and the tubular diaphysis is termed the metaphysis. -

Bone Cartilage Dense Fibrous CT (Tendons & Nonelastic Ligaments) Dense Elastic CT (Elastic Ligaments)
Chapter 6 Content Review Questions 1-8 1. The skeletal system consists of what connective tissues? Bone Cartilage Dense fibrous CT (tendons & nonelastic ligaments) Dense elastic CT (elastic ligaments) List the functions of these tissues. Bone: supports the body, protects internal organs, provides levers on which muscles act, store minerals, and produce blood cells. Cartilage provides a model for bone formation and growth, provides a smooth cushion between adjacent bones, and provides firm, flexible support. Tendons attach muscles to bones and ligaments attach bone to bone. 2. Name the major types of fibers and molecules found in the extracellular matrix of the skeletal system. Collagen Proteoglycans Hydroxyapatite Water Minerals How do they contribute to the functions of tendons, ligaments, cartilage and bones? The collagen fibers of tendons and ligaments make these structures very tough, like ropes or cables. Collagen makes cartilage tough, whereas the water-filled proteoglycans make it smooth and resistant. As a result, cartilage is relatively rigid, but springs back to its original shape if it is bent or slightly compressed, and it is an excellent shock absorber. The extracellular matrix of bone contains collagen and minerals, including calcium and phosphate. Collagen is a tough, ropelike protein, which lends flexible strength to the bone. The mineral component gives the bone compression (weight-bearing) strength. Most of the mineral in the bone is in the form of hydroxyapatite. 3. Define the terms diaphysis, epiphysis, epiphyseal plate, medullary cavity, articular cartilage, periosteum, and endosteum. Diaphysis – the central shaft of a long bone. Epiphysis – the ends of a long bone. Epiphyseal plate – the site of growth in bone length, found between each epiphysis and diaphysis of a long bone and composed of cartilage. -

BONE TISSUE Osteoblasts
Department of Histology and Embryology, P. J. Šafárik University, Medical Faculty, Košice BONE TISSUE: Sylabus for foreign students Author: MVDr. Zuzana Jonecová, CSc. Revised by: prof. MUDr. Eva Mechírová, CSc. BONE TISSUE - structural component of bones - specialized connective tissue for support and protection - composition: - cells - mineralized intercellular matrix – bone matrix Histological composition of the bone tissue: 1. Bone matrix – intercellular substance a. Inorganic components → Ca, P, Mg, K, Na → hyproxyapatit crystals (65%) crystals lie alongside collagen fibrills and are surrounded by amorphous ground substance b. Organic components: Fibers : collagen fibers (collagen type I ) ! Amorphous ground substance : noncollagenous proteins GAG, PG, structural GP small regulatory proteins 2. Cells a. Osteoprogenitor cells (preosteoblasts) b. Osteoblasts (bone forming cells) c. Osteocytes (inactive osteoblast) d. Osteoclasts (bone resorbing cells) Osteoblasts – line endosteal and periosteal surfaces ( 1 layer) - cuboidal cells with basophilic cytoplasm (proteosynthesis) - deposit of unmineralized organic matrix –osteoid – produced at the surface of the bone tissue when new bone tissue is required FUNCTION : 1) synthesis of organic components of bone matrix (growth, response to fracture, remodelling) 2) regulate mineralization of bone matrix 3) regulate activity of bone resorption (osteoclasts) Produce : - collagen type I (95 %) - non-collagenous bone matrix proteins : GAG, proteoglycans (hyaluronan, chondroitin and keratan sulfate) -

Functions of the Haversian System
Functions of the Haversian System DONALD H. ENLOW Department of Anatomy, The University of Michigan, Ann Arbor, Michigan The Haversian system or osteone has associated with areas of re-location in mus- been traditionally adopted as a universal cle attachment on a growing bone, and in unit of structure in compact bone. The remodeling processes involving resorption basic functions and the structural signifi- of periosteal bone surfaces during nieta- cance of primary and secondary Haversian physeal reduction in diameter axd re- tissue, however, are poorly understood. gional changes in shape. The hypothesis Two explanations on the functional mean- is advanced that this type of Haversian ing of the secondary Haversian system system functions as an anchoring mecha- have been proposed. These are (a) the nism which can maintain muscle con- interpretation of the osteone as an ex- tinuity and attachment with bone during clusive response to stress, and (b) the such remodeling changes. All secondary interpretation of the secondary osteone as osteones, regardless of particular function, an exclusive structural result of mineral are structurally comparable and represent mobilization and redistribution. However, a product of internal reconstruction with- the characteristic absence of the Haver- in compact bone. sian system in the compact bone of many History. Leeuwenhoek (1678) was the vertebrate species, including widely used first to notice the microscopic canal sgs- experimental forms such as the white rat, tern in bone, and he reported his observa- and the characteristic patterns of distribu- tions to members of the Royal Society in tion of Haversian systems in the bone of a series of personal communications which those species which do possess these were later published. -

Investigation of Physiochemical Properties of a Novel Gradient
Wayne State University Wayne State University Theses 1-1-2013 Investigation Of Physiochemical Properties Of A Novel Gradient Calcium Polyphosphate Bone Scaffold And Its Influence On Cellular Behavior Liang Chen Wayne State University, Follow this and additional works at: http://digitalcommons.wayne.edu/oa_theses Part of the Biomedical Engineering and Bioengineering Commons Recommended Citation Chen, Liang, "Investigation Of Physiochemical Properties Of A Novel Gradient Calcium Polyphosphate Bone Scaffold And Its Influence On Cellular Behavior" (2013). Wayne State University Theses. Paper 292. This Open Access Thesis is brought to you for free and open access by DigitalCommons@WayneState. It has been accepted for inclusion in Wayne State University Theses by an authorized administrator of DigitalCommons@WayneState. INVESTIGATION OF PHYSIOCHEMICAL PROPERTIES OF A NOVEL GRADIENT CALCIUM POLYPHOSPHATE BONE SCAFFOLD AND ITS INFLUENCE ON CELLULAR BEHAVIOR by Liang Chen THESIS Submitted to the Graduate School of Wayne State University Detroit, Michigan in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE 2013 MAJOR: BIOMEDICAL ENGINEERING ___________________________________ Advisor Date ACKNOWLEDGEMENTS I would like to especially thank Dr. Weiping Ren for granting me this great opportunity and support. I also thank Dr. Haward Mathew for his adviser and guidance on bioreactor system design and experiments. ii TABLE OF CONTENTS ACKNOWLEDGEMENTS ......................................................................................................................... -

A Comparison of the Microarchitecture of Lower Limb Long Bones Between Some Animal Models and Humans: a Review
Veterinarni Medicina, 58, 2013 (7): 339–351 Review Article A comparison of the microarchitecture of lower limb long bones between some animal models and humans: a review V.J. Cvetkovic1, S.J. Najman2, J.S. Rajkovic1, A.Lj. Zabar1, P.J. Vasiljevic1, Lj.B. Djordjevic1, M.D. Trajanovic3 1Faculty of Sciences and Mathematics, University of Nis, Nis, Serbia 2Faculty of Medicine, University of Nis, Nis, Serbia 3Faculty of Mechanical Engineering, University of Nis, Nis, Serbia ABSTRACT: Animal models are unavoidable and indispensable research tools in the fields of bone tissue engi- neering and experimental orthopaedics. The fact that there is not ideal animal model as well as the differences in the bone microarchitecture and physiology between animals and humans are complicate factors and make model implementation difficult. Therefore, the tendency should be directed towards extrapolation of the results from one animal model to another or from animal model to humans. So far, this is the first paper which provides an overview on the microarchitecture of lower limb long bones and discusses data related to osteon diameter, osteon canal diameter and their orientation, as well as intracortical canals and trabecular tissue microarchitecture in commonly used animal models compared to humans depending on age, gender and anatomical location of the bone. Understanding the differences between animal model and human bone microarchitecture should enable a more accurate extrapolation of experimental results from one animal model to another or from animal models to humans in the fields of bone tissue engineering and experimental orthopaedics. Also, this should be helpful in making decisions on which animal models are the most suitable for particular preclinical testing. -

Histomorphometric Analysis of the Variability of the Human Skeleton: Forensic Implications
Histomorphometric analysis of the variability of the human skeleton: forensic implications Marco Cummaudo1,2 PhD, Caterina Raffone MSc1, Annalisa Cappella1 PhD, Nicholas Márquez-Grant2 PhD, Cristina Cattaneo1 MSc, MD, PhD 1 LABANOF (Laboratorio di Antropologia e Odontologia Forense) Dipartimento di Scienze Biomediche per la Salute, via Mangiagalli 37, 20133, Università degli Studi di Milano, Italy 2 Cranfield Forensic Institute, Cranfield University, Defence Academy of the United Kingdom, Shrivenham, UK SN6 8LA Corresponding author: Marco Cummaudo - LABANOF (Laboratorio di Antropologia e Odontologia Forense) Dipartimento di Scienze Biomediche per la Salute, Via Mangiagalli n.37, 20133 University of Milan, Italy. Phone number: +393408009986; email address: [email protected] ORCID ID: 0000-0002-1173-9041 Declarations of interest: none Abstract In the last decades, the histomorphometric analysis of bone tissue has been utilized to develop equations for species discrimination of fragmentary bone. Although this technique showed promising results, its main limitation concerns the lack of knowledge on the histomorphometric variability which may exist between different bones of the skeleton. In a previous study, we demonstrated a significant histomorphological variability in different bones of the same individual and even in different sections of the same bone. The present study aimed at investigating the extent of intra- individual variability in bone histomorphometry throughout the human adult skeleton and areas of a single bone. Samples were taken along an entire medieval male adult human skeleton (aged between 26-45 years), including long, flat, irregular and sesamoid bones for a total of 49 cross-sections. The histomorphometric analysis revealed that the size of both Haversian systems and Haversian canals were statistically significantly larger in long and irregular bones compared to flat bones. -

S. S. College, Jehanabad Department: Zoology Class: M.Sc
S. S. College, Jehanabad Department: Zoology Class: M.Sc. Semester II Subject: Zoology Topic: Histology of mammalian bone Mode of teaching: Google classroom & WhatsApp Date & Time: 01.10.2020 & 10:30 Teacher: Praveen Deepak To join Department’s group, students can use following link https://chat.whatsapp.com/EHuHNfQzoAzJBMFNJvsjQx or scan QR Code WhatsApp No.: +91 75360 68068 HISTOLOGY OF MAMMALIAN BONES ____________________________________________________________ Bone is living tissue that makes up the skeleton of body. Being structural and anatomical component of skeleton, it provides support and protects the body and its organs like brain through skull, spinal cord through vertebrae, heart and lungs through rib cage, etc. Thus, functionally it assumes a significant mechanical role by the skeleton, and represents a stock of mineral salts to mobilize for maintenance of calcium and phosphorus homeostasis. Through the medullary spaces, it hosts, the bone produce various blood cells, store minerals, and provides structural and functional support for hematopoiesis. As it is a specialized form of connective tissue characterized by mineralized extracellular matrix, it contains – − Minerals: Minerals present in the bone are; o Calcium phosphate in the form of hydroxyapatite crystals [Ca 10 (PO 4)6 (OH) 2] o Calcium Carbonate (CaCO 3) o Magnesium Hydroxide: Mg(OH) 2 o Fluoride and Sulfate − Matrix: Mainly collagen (type I, VI) along with other matrix proteins. All collagen molecules are ~ 90% of total weight of bone matrix Functions of bone can be summarized as follows; − Storage for elements and minerals: homeostatic regulation of blood calcium levels − Mechanical structures for movement and protection of viscera − A home for hematopoietic tissue, and − Storage of adipose tissue: yellow marrow Bone tissue Bone tissue is also called as osseous tissue. -

Sistema Universitario Ana G. Méndez, Inc. School for Professional Studies
Sistema Universitario Ana G. Méndez, Inc. School for Professional Studies Florida Campuses Universidad del Este, Universidad Metropolitana, and Universidad del Turabo BIOL 303-Lab Human Biology I: Anatomy Laboratory Biología Humana I: Laboratorio de Anatomía © Sistema Universitario Ana G. Méndez, Inc. 2017 Derechos Reservados BIOL 303 HUMAN BIOLOGY LAB I 2 TABLA DE CONTENIDO/TABLE OF CONTENTS Página/Page GUÍA DE ESTUDIO ...................................................................................................................... 3 STUDY GUIDE .............................................................................................................................. 7 TALLER UNO .............................................................................................................................. 13 WORKSHOP TWO ...................................................................................................................... 21 TALLER TRES ............................................................................................................................. 30 WORKSHOP FOUR ..................................................................................................................... 36 TALLER CINCO .......................................................................................................................... 44 WORKSHOP SIX ......................................................................................................................... 56 TALLER SIETE ........................................................................................................................... -

Peter Takizawa Department of Cell Biology •Bone Structure and Composition
Bone Peter Takizawa Department of Cell Biology •Bone structure and composition •Bone cells •Bone modeling and remodeling •Regulation of osteoclast activation Bone serves mechanical, metabolic and cellular functions. Bone server a variety of important functions. It’s most familiar role is providing mechanical support and protection to organisms. It also serves as a repository for calcium. Bone houses bone marrow where most blood cells diferentiate from stem cells. These include red blood cells and white blood cells of the immune system. Mechanical bone consists of two architectures: compact and trabecular. Trabecular bone Compact bone Kerr Atlas of Functional Histology 1st edition Most bone comes in two architectures, both of which are seen in this section of long bone. On the outer surface is compact bone that appears as a dense wall of bone but compact contains blood vessels and nerves. In the interior is trabecular bone that consists of network of bone struts. Trabecular bone reduces the weight of bone while still providing robust mechanical support. It also allows space for bone marrow and diferentiation of blood cells. Because we are focusing on the mechanical functions of bone, it’s important to appreciate the structure and sections of long bone that are the primary load-bearing bones in the body. Long bones, as the name implies, are longer in one direction and consist of a shaft with two knob-like structures at either end. The shaft is called the diaphysis and the ends epiphysis. The outer portion of long bone is all compact bone and the inner portion is trabecular. -

Basic Science of Osteoarthritis Magali Cucchiarini1*, Laura De Girolamo2, Giuseppe Filardo3, J
Cucchiarini et al. Journal of Experimental Orthopaedics (2016) 3:22 Journal of DOI 10.1186/s40634-016-0060-6 Experimental Orthopaedics MEETING REPORT Open Access Basic science of osteoarthritis Magali Cucchiarini1*, Laura de Girolamo2, Giuseppe Filardo3, J. Miguel Oliveira4,5, Patrick Orth1,6, Dietrich Pape7,8 and Pascal Reboul9 Abstract Osteoarthritis (OA) is a prevalent, disabling disorder of the joints that affects a large population worldwide and for which there is no definitive cure. This review provides critical insights into the basic knowledge on OA that may lead to innovative end efficient new therapeutic regimens. While degradation of the articular cartilage is the hallmark of OA, with altered interactions between chondrocytes and compounds of the extracellular matrix, the subchondral bone has been also described as a key component of the disease, involving specific pathomechanisms controlling its initiation and progression. The identification of such events (and thus of possible targets for therapy) has been made possible by the availability of a number of animal models that aim at reproducing the human pathology, in particular large models of high tibial osteotomy (HTO). From a therapeutic point of view, mesenchymal stem cells (MSCs) represent a promising option for the treatment of OA and may be used concomitantly with functional substitutes integrating scaffolds and drugs/growth factors in tissue engineering setups. Altogether, these advances in the fundamental and experimental knowledge on OA may allow for the generation of improved, adapted therapeutic regimens to treat human OA. Keywords: Osteoarthritis, Articular cartilage, Bone, Animal models, Pathomechanisms, Interface, Stem cells, Tissue engineering Introduction and obesity. Knee OA affects over 70 million Europeans, Osteoarthritis (OA) is a systemic, chronic joint disorder and the direct costs exceed 2 billion euros. -

Compact Bone Spongy Bone Lamella (B) Bone Matrix Central (Haversian
2/7/20 (b) Flat bone (sternum) (a) Long bone (humerus) Spongy bone (d) Irregular bone (vertebra), Compact right lateral view (c) Short bone (talus) bone © 2018 Pearson Education, Inc. 1 © 2018 Pearson Education, Inc. 2 Articular cartilage Proximal epiphysis Spongy bone Epiphyseal line Periosteum Trabeculae of spongy bone Compact bone Osteon (Haversian Perforating Medullary (Volkmann’s) cavity (lined system) by endosteum) canal Blood vessel continues Diaphysis into medullary cavity containing marrow Blood vessel Lamellae Compact bone Central (Haversian) canal Perforating (Sharpey’s) fibers Periosteum Distal Periosteal epiphysis blood vessel (a) (a) © 2018 Pearson Education, Inc. 3 © 2018 Pearson Education, Inc. 4 Lamella Osteon Osteocyte Interstitial lamellae Canaliculus Lacuna Lacuna Central Central Bone matrix (Haversian) canal (Haversian) canal (b) (c) © 2018 Pearson Education, Inc. 5 © 2018 Pearson Education, Inc. 6 2/7/20 Articular cartilage Bone growth Hyaline Spongy cartilage bone Bone grows in length because: New center of bone growth New bone Articular cartilage Epiphyseal forming 1 Cartilage plate grows here. cartilage Growth Epiphyseal plate Medullary in bone cavity width 2 Cartilage is replaced Bone starting Invading by bone here. to replace Growth blood cartilage in bone vessels length 3 Cartilage New bone grows here. Bone collar forming Hyaline Epiphyseal cartilage plate cartilage model In an embryo In a fetus In a child © 2018 Pearson Education, Inc. 7 © 2018 Pearson Education, Inc. 8 Bone remodeling Growing shaft is remodeled as: Hematoma Articular cartilage External Bony callus callus of spongy Epiphyseal plate bone New Internal blood Healed 1 Bone is callus vessels resorbed by (fibrous fracture tissue and Spongy osteoclasts here.