85243382.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Elemental Fluorine Product Information (Pdf)
Elemental Fluorine Contents 1 Introduction ............................................................................................................... 4 2.1 Technical Application of Fluorine ............................................................................. 5 2.2 Electronic Application of Fluorine ........................................................................... 7 2.3 Fluorine On-Site Plant ............................................................................................ 8 3 Specifications ............................................................................................................ 9 4 Safety ...................................................................................................................... 10 4.1 Maintenance of the F2 system .............................................................................. 12 4.2 First Aid ................................................................................................................ 13 5.1 Chemical Properties ............................................................................................. 14 5.2 Physical Data ....................................................................................................... 15 6 Toxicity .................................................................................................................... 18 7 Shipping and Transport ........................................................................................... 20 8 Environment ........................................................................................................... -

2018 Annual Survey of Biological and Chemical Agents Regulated by Homeland Security (And Carcinogens Regulated by OSHA)
Name: Dept: Date: 2018 Annual Survey of Biological and Chemical Agents regulated by Homeland Security (and carcinogens regulated by OSHA) Due (date) All labs that do not have a current chemical inventory in Chematix MUST complete this survey. The University is required to make an annual report of all chemicals on the Chemical Facility Anti-Terrorism Standards (CFATS) lists. Additional information regarding the regulations is available on the EH&S website at http://www.safety.rochester.edu/restricted/occsafe/chemicalagent.html and https://www.selectagents.gov. 1. Please review the lists on the following pages and indicate if any are possessed by your lab. The CAS# has been added to the list for ease of searching databases. The CAS# is a Chemical Abstract Service numbering system which assigns a unique number to every chemical substance based on structure; this helps avoid confusion by use of synonyms or different naming conventions. a. If yes for possession, place an X in the applicable box and if requested, include the quantity held in your lab. b. If no, leave blank. 2. After reviewing the list, please complete the information box below (or on last page for possession), then sign, date and return to EH&S. 3. Please call Donna Douglass at 275-2402 if you have any questions. Thank you for your cooperation in collecting data required by the Department of Homeland Security! Possession: 1) Fill in applicable boxes, 2) have PI sign last page, 3) return all pages to Donna Douglass OR Non-possession: 1) Check only one box on the left, 2) sign, 3) return just this page to Donna Douglass I do not have a lab, do not work in a lab, nor do I possess any of the agents in this survey. -

Material Safety Data Sheet Prepared to US OSHA, CMA, ANSI, Canadian WHMIS and EU Standards
MSDS NUMBER: 1201 Fluorine Excimer Laser Mix (1.0% or less Fluorine) Material Safety Data Sheet Prepared to US OSHA, CMA, ANSI, Canadian WHMIS and EU Standards SECTION 1. PRODUCT IDENTIFICATION PRODUCT NAME: Fluorine Excimer Laser Mix (1.0% or less Fluorine) US DOT NAME: Compressed Gas, n.o.s. (Fluorine, other gas) UN 1956 (see Sec. 14) CHEMICAL NAME: Mixture of Fluorine (1.0% or Less) and Argon, Krypton or Xenon balance Helium, Neon or Nitrogen FORMULA: Argon = Ar; Fluorine = F2; Helium = He; Krypton = Kr; Neon = Ne; Nitrogen = N2; SYNONYMS: Not Applicable US MANUFACTURER: Linde LLC 575 Mountain Ave. Murray Hill, NJ 07974 Phone: 908-464-8100 lindeus.com 24 HOUR EMERGENCY CONTACT, CHEMTREC: 800/424-9300, 703/527-3887 For additional product information contact your customer service representative. PRODUCT USE: In Excimer Laser and Research and Development ALL WHMIS required information is included in appropriate sections based on the ANSI Z400.1-2004 format. This product has been classified in accordance with the hazard criteria of the CPR and the MSDS contains all the information required by the CPR. The product is also classified per all applicable EU Directives through EC 1907: 2006 SECTION 2. HAZARD IDENTIFICATION EU LABELING AND CLASSIFICATION: This gas mixture meets the definition of hazardous according to the criteria of the European Union Council Directive 67/548/EEC and based on current guidelines under EC 1907: 2006. EU CLASSIFICATION: Xn (Harmful) EU RISK PHRASES: R: 20; R: 21; R: 36/37/38 EU SAFETY PHRASES: S: (1/2)*; S: 7/9, S: 26, S: 36/37/39, S: 45 See Section 15 for full definition of Risk and Safety Phrases. -

Chemical Chemical Hazard and Compatibility Information
Chemical Chemical Hazard and Compatibility Information Acetic Acid HAZARDS & STORAGE: Corrosive and combustible liquid. Serious health hazard. Reacts with oxidizing and alkali materials. Keep above freezing point (62 degrees F) to avoid rupture of carboys and glass containers.. INCOMPATIBILITIES: 2-amino-ethanol, Acetaldehyde, Acetic anhydride, Acids, Alcohol, Amines, 2-Amino-ethanol, Ammonia, Ammonium nitrate, 5-Azidotetrazole, Bases, Bromine pentafluoride, Caustics (strong), Chlorosulfonic acid, Chromic Acid, Chromium trioxide, Chlorine trifluoride, Ethylene imine, Ethylene glycol, Ethylene diamine, Hydrogen cyanide, Hydrogen peroxide, Hydrogen sulfide, Hydroxyl compounds, Ketones, Nitric Acid, Oleum, Oxidizers (strong), P(OCN)3, Perchloric acid, Permanganates, Peroxides, Phenols, Phosphorus isocyanate, Phosphorus trichloride, Potassium hydroxide, Potassium permanganate, Potassium-tert-butoxide, Sodium hydroxide, Sodium peroxide, Sulfuric acid, n-Xylene. Acetone HAZARDS & STORAGE: Store in a cool, dry, well ventilated place. INCOMPATIBILITIES: Acids, Bromine trifluoride, Bromine, Bromoform, Carbon, Chloroform, Chromium oxide, Chromium trioxide, Chromyl chloride, Dioxygen difluoride, Fluorine oxide, Hydrogen peroxide, 2-Methyl-1,2-butadiene, NaOBr, Nitric acid, Nitrosyl chloride, Nitrosyl perchlorate, Nitryl perchlorate, NOCl, Oxidizing materials, Permonosulfuric acid, Peroxomonosulfuric acid, Potassium-tert-butoxide, Sulfur dichloride, Sulfuric acid, thio-Diglycol, Thiotrithiazyl perchlorate, Trichloromelamine, 2,4,6-Trichloro-1,3,5-triazine -

19770005666.Pdf
General Disclaimer One or more of the Following Statements may affect this Document This document has been reproduced from the best copy furnished by the organizational source. It is being released in the interest of making available as much information as possible. This document may contain data, which exceeds the sheet parameters. It was furnished in this condition by the organizational source and is the best copy available. This document may contain tone-on-tone or color graphs, charts and/or pictures, which have been reproduced in black and white. This document is paginated as submitted by the original source. Portions of this document are not fully legible due to the historical nature of some of the material. However, it is the best reproduction available from the original submission. Produced by the NASA Center for Aerospace Information (CASI) V NASA TECHNICAL NASA TM X-73983 MEMORANDUM 00 X I-- A SURVEY OF KINETIC DATA OF COMPOUNDS CONTAINING FLUORINE Dana A. Brewer Langley Research Center (NASA-TM-X-73983) A SURVEY OF KINETIC DATA N77-12609 OF COMPOUNDS CONTAINING FLOURINE (NASA) 119 p, HC A06/MF A01 CSCL 04A Unclas G3/46 55838 November 1976 This informal documentation medium is used to provide accelerated or special release of technical information to selected users. The contents may not meet NASA formal editing and publication standards, may be re- vised, or may I be incorporated in another publication. 4WAil NATIONAL AERONAUTICS AND SPACE ADMINISTRATION DEC 1976 LANGLEY RESEARCH CENTER, HAMPTON, VIRGINIA 23665 WEIVED 0M tosp, Sn PACILITY INpUT BRANCH < C' ' r ^| ' 1. Report No. -

Silica Material Safety Data Sheet
Distributed by: u.s. SILICA COMPANY Laguna Clay Company 14400 Lomitas Ave City of Industry, CA 91746 Material Safety Data Sheet 1-800-4Laguna Page 1 of 11 [email protected] • - www.lagunaclay.com Product Name: Silica Sand and Ground Silica Product Description: Crystalline Silica 11. Identification of the substance/preparation and of the company/undertaking 1.1. Identification of the substance or preparation Product Name/Trade Names: Sand and Ground Silica Sand (flour) sold under various names: ASTM TESTING SANDS, GLASS SAND' FLINT SILICA' DM-SERIES • F-SERIES • FOUNDRY SANDS· FJ-SERIES • H-SERIES' L-SERIES • N-SERIES' NJ SERIES, OK-SERIES' P-SERIES· T-SERIES' HYDRAULIC FRACING SANDS, MIN-U-SIL® Fine Ground Silica' MYSTIC WHITE® • #1 DRY' #1 SPECIAL' PENN SAND® • Q-ROK® • SIL-CO-SIL® Ground Silica' Supersil® • MASON SAND· GS SERIES' PER-SPEC Chemical Name or Synonym: Silicon Dioxide (SiOz). Sand, Silica Sand, Quartz, Crystalline Silica, Flint, Ground Silica (flour). White or tan sand or ground silica with no odor. 1.2. Use of the Substance / Preparation Main Applications (non-exhaustive list): abrasives, brick, ceramics, foundry castings, glass, grout, hydraulic frac (proppant) sand, mortar, paint and coatings, silicate chemistry, silicone rubber, thermoset plastics. 1.3. Company / Producer U.S. Silica Company P.O. Box 187 106 Sand Mine Road Berkeley Springs, West Virginia 25411 U.S.A. Phone: 304-258-2500 Emergency Phone: 304-258-2500 Fax: 304-258-8295 1 2. Hazards Identification 2.1. EMERGENCY OVERVIEW: The U. S. Silica Company material is a white or tan sand, or ground sand. It is not flammable, combustible or explosive. -

High Hazard Chemical Policy
Environmental Health & Safety Policy Manual Issue Date: 2/23/2011 Policy # EHS-200.09 High Hazard Chemical Policy 1.0 PURPOSE: To minimize hazardous exposures to high hazard chemicals which include select carcinogens, reproductive/developmental toxins, chemicals that have a high degree of toxicity. 2.0 SCOPE: The procedures provide guidance to all LSUHSC personnel who work with high hazard chemicals. 3.0 REPONSIBILITIES: 3.1 Environmental Health and Safety (EH&S) shall: • Provide technical assistance with the proper handling and safe disposal of high hazard chemicals. • Maintain a list of high hazard chemicals used at LSUHSC, see Appendix A. • Conduct exposure assessments and evaluate exposure control measures as necessary. Maintain employee exposure records. • Provide emergency response for chemical spills. 3.2 Principle Investigator (PI) /Supervisor shall: • Develop and implement a laboratory specific standard operation plan for high hazard chemical use per OSHA 29CFR 1910.1450 (e)(3)(i); Occupational Exposure to Hazardous Chemicals in Laboratories. • Notify EH&S of the addition of a high hazard chemical not previously used in the laboratory. • Ensure personnel are trained on specific chemical hazards present in the lab. • Maintain Material Safety Data Sheets (MSDS) for all chemicals, either on the computer hard drive or in hard copy. • Coordinate the provision of medical examinations, exposure monitoring and recordkeeping as required. 3.3 Employees: • Complete all necessary training before performing any work. • Observe all safety -

Material Safety Data Sheet
SAFETY DATA SHEET FILE NO.: SDS.NATURALSTONE.01 NATURAL STONE SDS DATE: January 2015 SECTION 1: IDENTIFICATION PRODUCT NAME: Natural Stone Products (Tiles, Trim, Decoration) SYNONYMS: Marble, Onyx, Travertine, Limestone PRODUCT CODES: All current product references PRODUCT USE: Residential & Commercial Construction Applications CHEMICAL NAME: N/A CHEMICAL FAMILY: Inorganic Compound CHEMICAL FORMULA: Natural occurring Calcium Carbonate Mixture MANUFACTURER: Anatolia Tile & Stone (Designer & Importer) DIVISION: Corporate Office ADDRESS: 8300 Huntington Road, Vaughan, ON Canada L4L 1A5 EMERGE NCY PHONE: 905-771-3800 CHEMTREC PHONE: OTHER CALLS: FAX PHONE: 905-771-6300 PREPARED BY: Diana Vacca SECTION 2: HAZARD(S) IDENTIFICATION CARCINOGEN IRRITANT / REPIRATORY TRACT IRRITATION NOTES: Not applicable for intact stone products. Excessive exposure to dust can cause discomfort and mechanical irritation. Long term exposure to silica dusts can lead to silicosis. EMERGENCY OVERVIEW: OSHA LISTS THESE AS CATEGORY “B” STONES (CONTAINING LESS THAN 1% CRYSTALLINE SILICA), CONSIDRED TO BE A NUISANCE PARTICULATE FROM DUST THAT CAN ACCUMULATE IN THE LUNGS. AVOID DUST PRODUCTION BY CUTTING UNDER WATER, AND USE PROTECTIVE BREATHING APPARATUS AND EYE PROTECTION, AS OUTLINED IN SECTION 8. POTENTIAL HEALTH EFFECTS EYES: Mechanical stress through eye rubbing if exposed to dust or airborne particulates SKIN: Skin irritation if exposed to dust or airborne particulates INGESTION: Potential choking hazard if exposed to airborne particulates INHALATION: Mechanical stress from inhalation of dust or airborne particulates ACUTE HEALTH HAZARDS: No further relevant information available. CHRONIC HEALTH HAZARDS: No further relevant information available PAGE 1 OF 8 SAFETY DATA SHEET FILE NO.: SDS.NATURALSTONE.01 NATURAL STONE SDS DATE: January 2015 SECTION 2: HAZARD(S) IDENTIFICATION - CONTINUED MEDICAL CONDITIONS GENERALLY AGGRAVATED BY EXPOSURE: No further relevant information available CARCINOGENICITY *: The composition of SiO 2 may be up to 100% crystalline silica. -

Fluorine Flame Calorimetry. III. the Heat of Formation of Chlorine
JOURNAL OF RESEARCH of the National Bureau of Standards - A. Physic s and Chemistry Vol. 74A, No.6, November- December 1970 Fluorine Flame Calorimetry III. The Heat of Formation of Chlorine Trifluoride at 298.15 K* R. C. King** and G. T. Armstrong Institute for Materials Research, National Bureau of Standards, Washington, D.C. 20234 (July 6, 1970) The standard heat 0(" formati on of chlorine triA uoride (gas) at 298.15 K has been determined to be - 164.65 kJ mol- I (- 39.35 kcal mol- I) with a n ove rall experimenta l uncertainty of 5. 14 kJ mol - I (1.23 kcal mol- I). This valu e is de rived from the enthalpies of the foll owing reactions whic h were measured directly in a flam e calorimeter operated at 1 atm pressure a nd 303.5 K, together with data from previous investigations. C!Fig) + 2H,(g) + 100H, O(l) -> [H CI·3HF·lOOH, OI(l) (1/2)C I2(g) + 1/2H,(g) + [3HF·100H, OJ(l ) -> [HC I·3 HF·100H,OI(l ) The e nthalp y of formation of [HCI· lOOH ,O](I) was a lso measured. The average CI-F bond e ne rgy in c hl orine triAuoride is calc ulated to be 160.1 kJ mol- I (38.26 kcal mol- I). Key wo rds: Bond e nergy (CI-F); chlorine reaction with hydrogen ; chl ori ne trifluoride, heat of forma· tion; fl ame calorim etry; fl ow calorime try; flu orine compounds; heat of formati on; heat of reacti on ; hydrogen chl oride (aqueous), he at of form ation; mixed acids: (HCl + 3HFL", heat of formation; reaction c alorimetry; reacti on with h ydrogen. -

List of Reactive Chemicals
LIST OF REACTIVE CHEMICALS Chemical Prefix Chemical Name Reactive Reactive Reactive CAS# Chemical Chemical Chemical Stimulus 1 Stimulus 2 Stimulus 3 111-90-0 "CARBITOL" SOLVENT D 111-15-9 "CELLOSOLVE" ACETATE D 110-80-5 "CELLOSOLVE" SOLVENT D 2- (2,4,6-TRINITROPHENYL)ETHYL ACETATE (1% IN ACETONE & BENZENE S 12427-38-2 AAMANGAN W 88-85-7 AATOX S 40487-42-1 AC 92553 S 105-57-7 ACETAL D 75-07-0 ACETALDEHYDE D 105-57-7 ACETALDEHYDE, DIETHYL ACETAL D 108-05-4 ACETIC ACID ETHENYL ESTER D 108-05-4 ACETIC ACID VINYL ESTER D 75-07-0 ACETIC ALDEHYDE D 101-25-7 ACETO DNPT T 126-84-1 ACETONE DIETHYL ACETAL D 108-05-4 ACETOXYETHYLENE D 108-05-4 1- ACETOXYETHYLENE D 37187-22-7 ACETYL ACETONE PEROXIDE, <=32% AS A PASTE T 37187-22-7 ACETYL ACETONE PEROXIDE, <=42% T 37187-22-7 ACETYL ACETONE PEROXIDE, >42% T S 644-31-5 ACETYL BENZOYL PEROXIDE (SOLID OR MORE THAN 45% IN SOLUTION) T S 644-31-5 ACETYL BENZOYL PEROXIDE, <=45% T 506-96-7 ACETYL BROMIDE W 75-36-5 ACETYL CHLORIDE W ACETYL CYCLOHEXANE SULFONYL PEROXIDE (>82% WITH <12% WATER) T S 3179-56-4 ACETYL CYCLOHEXANE SULFONYL PEROXIDE, <=32% T 3179-56-4 ACETYL CYCLOHEXANE SULFONYL PEROXIDE, <=82% T 674-82-8 ACETYL KETENE (POISON INHALATION HAZARD) D 110-22-5 ACETYL PEROXIDE, <=27% T 110-22-5 ACETYL PEROXIDE, SOLID, OR MORE THAN 27% IN SOLUTION T S 927-86-6 ACETYLCHOLINE PERCHLORATE O S 74-86-2 ACETYLENE D 74-86-2 ACETYLENE (LIQUID) D ACETYLENE SILVER NITRATE D 107-02-08 ACRALDEHYDE (POISON INHALATION HAZARD) D 79-10-7 ACROLEIC ACID D 107-02-08 ACROLEIN, INHIBITED (POISON INHALATION HAZARD) D 107-02-08 ACRYLALDEHYDE (POISON INHALATION HAZARD) D 79-10-7 ACRYLIC ACID D 141-32-2 ACRYLIC ACID BUTYL ESTER D 140-88-5 ACRYLIC ACID ETHYL ESTER D 96-33-3 ACRYLIC ACID METHYL ESTER D Stimulus - Stimuli is the thermal, physical or chemical input needed to induce a hazardous reaction. -

Inorganic Chemistry
INORGANIC CHEMISTRY Saito Inorganic Chemistry Saito This text is disseminated via the Open Education Resource (OER) LibreTexts Project (https://LibreTexts.org) and like the hundreds of other texts available within this powerful platform, it freely available for reading, printing and "consuming." Most, but not all, pages in the library have licenses that may allow individuals to make changes, save, and print this book. Carefully consult the applicable license(s) before pursuing such effects. Instructors can adopt existing LibreTexts texts or Remix them to quickly build course-specific resources to meet the needs of their students. Unlike traditional textbooks, LibreTexts’ web based origins allow powerful integration of advanced features and new technologies to support learning. The LibreTexts mission is to unite students, faculty and scholars in a cooperative effort to develop an easy-to-use online platform for the construction, customization, and dissemination of OER content to reduce the burdens of unreasonable textbook costs to our students and society. The LibreTexts project is a multi-institutional collaborative venture to develop the next generation of open-access texts to improve postsecondary education at all levels of higher learning by developing an Open Access Resource environment. The project currently consists of 13 independently operating and interconnected libraries that are constantly being optimized by students, faculty, and outside experts to supplant conventional paper-based books. These free textbook alternatives are organized within a central environment that is both vertically (from advance to basic level) and horizontally (across different fields) integrated. The LibreTexts libraries are Powered by MindTouch® and are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. -
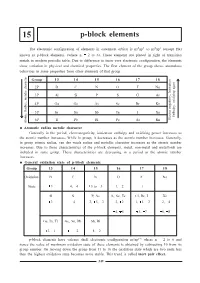
P-Block Elements
15 p-block elements The electronic configuration of elements in outermost orbital is ns2np1 to ns2np6 (except He) known as p-block elements. (where n = 2 to 6). These elements are placed in right of transition metals in modern periodic table. Due to difference in inner core electronic configuration, the elements show variation in physical and chemical properties. The first element of the group shows anomalous behaviour in some properties from other elements of that group. Group 13 14 15 16 17 18 2P BC NO F Ne 3P Al Si P S Cl Ar , Ionisation 4P Ga Ge As Se Br Kr , oxidizing agent 5P In Sn Sb Te I Xe Enthalpy 6P Tl Pb Bi Po At Rn Electro negativity Atomic Radius, metalic character Atomatic radius metalic character Generally in the period, electronegativity, ionization enthalpy and oxidising power increases as the atomic number increases. While in group, it decreases as the atomic number increases. Generally, in group atomic radius, van der waals radius and metallic character increases as the atomic number increases. Due to these characteristics of the p-block elements, metal, non-metal and metalloids are included in same group. These characteristics are decreasing in a period as the atomic number increases. General oxidation state of p-block elements Group 13 14 15 16 17 18 Oxidation BC NO F Ne State +3 +4, -4 +5 to -3 -1, -2 -1 - Al Si P, As S, Se, Te Cl, Br, I Xe +3 +4 +3, +5, -3 -2, +2 -1, +1, +3 +2, +4 +4, +6 +5, +7 +6, +8 Ga, In, Tl Ge, Sn, Pb Sb, Bi +3, +1 +4, +2 +4, +2 p-block elements have valence shell electronic configuration ns2np16 where n = 2 to 6 and hence the value of maximum oxidation state of these elements is obtained by subtracting 10 from its group number.