Analysis of Data Acquired Using ROC Paradigm and Its Extensions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
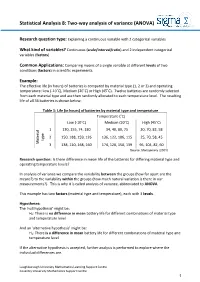
Statistical Analysis 8: Two-Way Analysis of Variance (ANOVA)
Statistical Analysis 8: Two-way analysis of variance (ANOVA) Research question type: Explaining a continuous variable with 2 categorical variables What kind of variables? Continuous (scale/interval/ratio) and 2 independent categorical variables (factors) Common Applications: Comparing means of a single variable at different levels of two conditions (factors) in scientific experiments. Example: The effective life (in hours) of batteries is compared by material type (1, 2 or 3) and operating temperature: Low (-10˚C), Medium (20˚C) or High (45˚C). Twelve batteries are randomly selected from each material type and are then randomly allocated to each temperature level. The resulting life of all 36 batteries is shown below: Table 1: Life (in hours) of batteries by material type and temperature Temperature (˚C) Low (-10˚C) Medium (20˚C) High (45˚C) 1 130, 155, 74, 180 34, 40, 80, 75 20, 70, 82, 58 2 150, 188, 159, 126 136, 122, 106, 115 25, 70, 58, 45 type Material 3 138, 110, 168, 160 174, 120, 150, 139 96, 104, 82, 60 Source: Montgomery (2001) Research question: Is there difference in mean life of the batteries for differing material type and operating temperature levels? In analysis of variance we compare the variability between the groups (how far apart are the means?) to the variability within the groups (how much natural variation is there in our measurements?). This is why it is called analysis of variance, abbreviated to ANOVA. This example has two factors (material type and temperature), each with 3 levels. Hypotheses: The 'null hypothesis' might be: H0: There is no difference in mean battery life for different combinations of material type and temperature level And an 'alternative hypothesis' might be: H1: There is a difference in mean battery life for different combinations of material type and temperature level If the alternative hypothesis is accepted, further analysis is performed to explore where the individual differences are. -

5. Dummy-Variable Regression and Analysis of Variance
Sociology 740 John Fox Lecture Notes 5. Dummy-Variable Regression and Analysis of Variance Copyright © 2014 by John Fox Dummy-Variable Regression and Analysis of Variance 1 1. Introduction I One of the limitations of multiple-regression analysis is that it accommo- dates only quantitative explanatory variables. I Dummy-variable regressors can be used to incorporate qualitative explanatory variables into a linear model, substantially expanding the range of application of regression analysis. c 2014 by John Fox Sociology 740 ° Dummy-Variable Regression and Analysis of Variance 2 2. Goals: I To show how dummy regessors can be used to represent the categories of a qualitative explanatory variable in a regression model. I To introduce the concept of interaction between explanatory variables, and to show how interactions can be incorporated into a regression model by forming interaction regressors. I To introduce the principle of marginality, which serves as a guide to constructing and testing terms in complex linear models. I To show how incremental I -testsareemployedtotesttermsindummy regression models. I To show how analysis-of-variance models can be handled using dummy variables. c 2014 by John Fox Sociology 740 ° Dummy-Variable Regression and Analysis of Variance 3 3. A Dichotomous Explanatory Variable I The simplest case: one dichotomous and one quantitative explanatory variable. I Assumptions: Relationships are additive — the partial effect of each explanatory • variable is the same regardless of the specific value at which the other explanatory variable is held constant. The other assumptions of the regression model hold. • I The motivation for including a qualitative explanatory variable is the same as for including an additional quantitative explanatory variable: to account more fully for the response variable, by making the errors • smaller; and to avoid a biased assessment of the impact of an explanatory variable, • as a consequence of omitting another explanatory variables that is relatedtoit. -

Analysis of Variance and Analysis of Variance and Design of Experiments of Experiments-I
Analysis of Variance and Design of Experimentseriments--II MODULE ––IVIV LECTURE - 19 EXPERIMENTAL DESIGNS AND THEIR ANALYSIS Dr. Shalabh Department of Mathematics and Statistics Indian Institute of Technology Kanpur 2 Design of experiment means how to design an experiment in the sense that how the observations or measurements should be obtained to answer a qqyuery inavalid, efficient and economical way. The desigggning of experiment and the analysis of obtained data are inseparable. If the experiment is designed properly keeping in mind the question, then the data generated is valid and proper analysis of data provides the valid statistical inferences. If the experiment is not well designed, the validity of the statistical inferences is questionable and may be invalid. It is important to understand first the basic terminologies used in the experimental design. Experimental unit For conducting an experiment, the experimental material is divided into smaller parts and each part is referred to as experimental unit. The experimental unit is randomly assigned to a treatment. The phrase “randomly assigned” is very important in this definition. Experiment A way of getting an answer to a question which the experimenter wants to know. Treatment Different objects or procedures which are to be compared in an experiment are called treatments. Sampling unit The object that is measured in an experiment is called the sampling unit. This may be different from the experimental unit. 3 Factor A factor is a variable defining a categorization. A factor can be fixed or random in nature. • A factor is termed as fixed factor if all the levels of interest are included in the experiment. -

THE ONE-SAMPLE Z TEST
10 THE ONE-SAMPLE z TEST Only the Lonely Difficulty Scale ☺ ☺ ☺ (not too hard—this is the first chapter of this kind, but youdistribute know more than enough to master it) or WHAT YOU WILL LEARN IN THIS CHAPTERpost, • Deciding when the z test for one sample is appropriate to use • Computing the observed z value • Interpreting the z value • Understandingcopy, what the z value means • Understanding what effect size is and how to interpret it not INTRODUCTION TO THE Do ONE-SAMPLE z TEST Lack of sleep can cause all kinds of problems, from grouchiness to fatigue and, in rare cases, even death. So, you can imagine health care professionals’ interest in seeing that their patients get enough sleep. This is especially the case for patients 186 Copyright ©2020 by SAGE Publications, Inc. This work may not be reproduced or distributed in any form or by any means without express written permission of the publisher. Chapter 10 ■ The One-Sample z Test 187 who are ill and have a real need for the healing and rejuvenating qualities that sleep brings. Dr. Joseph Cappelleri and his colleagues looked at the sleep difficul- ties of patients with a particular illness, fibromyalgia, to evaluate the usefulness of the Medical Outcomes Study (MOS) Sleep Scale as a measure of sleep problems. Although other analyses were completed, including one that compared a treat- ment group and a control group with one another, the important analysis (for our discussion) was the comparison of participants’ MOS scores with national MOS norms. Such a comparison between a sample’s mean score (the MOS score for par- ticipants in this study) and a population’s mean score (the norms) necessitates the use of a one-sample z test. -

ANALYSIS of VARIANCE and MISSING OBSERVATIONS in COMPLETELY RANDOMIZED, RANDOMIZED BLOCKS and LATIN SQUARE DESIGNS a Thesis
ANALYSIS OF VARIANCE AND MISSING OBSERVATIONS IN COMPLETELY RANDOMIZED, RANDOMIZED BLOCKS AND LATIN SQUARE DESIGNS A Thesis Presented to The Department of Mathematics Kansas State Teachers College, Emporia, Kansas In Partial Fulfillment of the Requirements for the Degree Master of Science by Kiritkumar K. Talati May 1972 c; , ACKNOWLEDGEMENTS Sincere thanks and gratitude is expressed to Dr. John Burger for his assistance, patience, and his prompt attention in all directions in preparing this paper. A special note of appreciation goes to Dr. Marion Emerson, Dr. Thomas Davis, Dr. George Poole, Dr. Darrell Wood, who rendered assistance in the research of this paper. TABLE OF CONTENTS CHAPTER I. INTRODUCTION • • • • • • • • • • • • • • • • 1 A. Preliminary Consideration. • • • • • • • 1 B. Purpose and Assumptions of the Analysis of Variance • • • • • • • • •• 1 C. Analysis of Covariance • • • • • • • • • 2 D. Definitions. • • • • • • • • • • • • • • ) E. Organization of Paper. • • • • • • • • • 4 II. COMPLETELY RANDOMIZED DESIGN • • • • • • • • 5 A. Description............... 5 B. Randomization. • • • • • • • • • • • • • 5 C. Problem and Computations •••••••• 6 D. Conclusion and Further Applications. •• 10 III. RANDOMIZED BLOCK DESIGN. • • • • • • • • • • 12 A. Description............... 12 B. Randomization. • • • • • • • • • • • • • 1) C. Problem and Statistical Analysis • • • • 1) D. Efficiency of Randomized Block Design as Compared to Completely Randomized Design. • • • • • • • • • •• 20 E. Missing Observations • • • • • • • • •• 21 F. -

Analysis of Variance in the Modern Design of Experiments
Analysis of Variance in the Modern Design of Experiments Richard DeLoach* NASA Langley Research Center, Hampton, Virginia, 23681 This paper is a tutorial introduction to the analysis of variance (ANOVA), intended as a reference for aerospace researchers who are being introduced to the analytical methods of the Modern Design of Experiments (MDOE), or who may have other opportunities to apply this method. One-way and two-way fixed-effects ANOVA, as well as random effects ANOVA, are illustrated in practical terms that will be familiar to most practicing aerospace researchers. I. Introduction he Modern Design of Experiments (MDOE) is an integrated system of experiment design, execution, and T analysis procedures based on industrial experiment design methods introduced at the beginning of the 20th century for various product and process improvement applications. MDOE is focused on the complexities and special requirements of aerospace ground testing. It was introduced to the experimental aeronautics community at NASA Langley Research Center in the 1990s as part of a renewed focus on quality and productivity improvement in wind tunnel testing, and has been used since then in numerous other applications as well. MDOE has been offered as a more productive, less costly, and higher quality alternative to conventional testing methods used widely in the aerospace industry, and known in the literature of experiment design as ―One Factor At a Time‖ (OFAT) testing. The basic principles of MDOE are documented in the references1-4, and some representative examples of its application in aerospace research are also provided5-16. The MDOE method provides productivity advantages over conventional testing methods by generating more information per data point. -

Analysis of Variance∗
Analysis of variance∗ Andrew Gelman† February 25, 2005 Abstract Analysis of variance (ANOVA) is a statistical procedure for summarizing a classical linear model—a decomposition of sum of squares into a component for each source of variation in the model—along with an associated test (the F-test) of the hypothesis that any given source of variation in the model is zero. When applied to generalized linear models, multilevel models, and other extensions of classical regression, ANOVA can be extended in two different directions. First, the F-test can be used (in an asymptotic or approximate fashion) to compare nested models, to test the hypothesis that the simpler of the models is sufficient to explain the data. Second, the idea of variance decomposition can be interpreted as inference for the variances of batches of parameters (sources of variation) in multilevel regressions. 1 Introduction Analysis of variance (ANOVA) represents a set of models that can be fit to data, and also a set of methods that can be used to summarize an existing fitted model. We shall first consider ANOVA as it applies to classical linear models (the context for which it was originally devised; Fisher, 1925) and then discuss how ANOVA has been extended to generalized linear models and multilevel models. Analysis of variance is particularly effective tool for analyzing highly structured experimental data (in agriculture, multiple treatments applied to different batches of animals or crops; in psychology, multi-factorial experiments manipulating several independent experimental conditions and applied to groups of people; industrial experiments in which multiple factors can be altered at different times and in different locations). -

Multi-Armed Rcts: a Design-Based Framework (2017–4027)
Analytic Technical Assistance and Development U.S. Department of Education August 2017 Multi-armed RCTs: A design- based framework Peter Z. Schochet Mathematica Policy Research, Inc. i NCEE 2017-4027 The National Center for Education Evaluation and Regional Assistance (NCEE) conducts unbiased, large-scale evaluations of education programs and practices supported by federal funds; provides research-based technical assistance to educators and policymakers; and supports the synthesis and the widespread dissemination of the results of research and evaluation throughout the United States. August 2017 This report was prepared for the Institute of Education Sciences (IES) by Decision Information Resources, Inc. under Contract ED-IES-12-C-0057, Analytic Technical Assistance and Development. The content of the publication does not necessarily reflect the views or policies of IES or the U.S. Department of Education nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This report is in the public domain. While permission to reprint this publication is not necessary, it should be cited as: Schochet, P.Z. (2017) Multi-armed RCTs: A design-based framework (2017–4027). Washington, DC: U.S. Department of Education, Institute of Education Sciences, National Center for Education Evaluation and Regional Assistance, Analytic Technical Assistance and Development. i Contents Topics on design-based causal inference for multi-armed RCTs ................................................................. -

One-Way Analysis of Variance: Comparing Several Means
One-Way Analysis of Variance: Comparing Several Means Diana Mindrila, Ph.D. Phoebe Balentyne, M.Ed. Based on Chapter 25 of The Basic Practice of Statistics (6th ed.) Concepts: . Comparing Several Means . The Analysis of Variance F Test . The Idea of Analysis of Variance . Conditions for ANOVA . F Distributions and Degrees of Freedom Objectives: Describe the problem of multiple comparisons. Describe the idea of analysis of variance. Check the conditions for ANOVA. Describe the F distributions. Conduct and interpret an ANOVA F test. References: Moore, D. S., Notz, W. I, & Flinger, M. A. (2013). The basic practice of statistics (6th ed.). New York, NY: W. H. Freeman and Company. Introduction . The two sample t procedures compare the means of two populations. However, many times more than two groups must be compared. It is possible to conduct t procedures and compare two groups at a time, then draw a conclusion about the differences between all of them. However, if this were done, the Type I error from every comparison would accumulate to a total called “familywise” error, which is much greater than for a single test. The overall p-value increases with each comparison. The solution to this problem is to use another method of comparison, called analysis of variance, most often abbreviated ANOVA. This method allows researchers to compare many groups simultaneously. ANOVA analyzes the variance or how spread apart the individuals are within each group as well as between the different groups. Although there are many types of analysis of variance, these notes will focus on the simplest type of ANOVA, which is called the one-way analysis of variance. -

Ztest — Z Tests (Mean-Comparison Tests, Known Variance)
Title stata.com ztest — z tests (mean-comparison tests, known variance) Description Quick start Menu Syntax Options Remarks and examples Stored results Methods and formulas References Also see Description ztest performs z tests on the equality of means, assuming known variances. The test can be performed for one sample against a hypothesized population value or for no difference in population means estimated from two samples. Two-sample tests can be conducted for paired and unpaired data. Clustered data are also supported. ztesti is the immediate form of ztest; see [U] 19 Immediate commands. For the comparison of means when variances are unknown, use ttest; see[ R] ttest. Quick start One-sample test that the mean of v1 is 3 at the 90% confidence level ztest v1 == 3, level(90) As above, and adjust for clustering with clusters defined by cvar and an intraclass correlation of 0.5 ztest v1 == 3, level(90) cluster(cvar) rho(0.5) Unpaired z test that the mean of v1 is equal between two groups defined by catvar ztest v1, by(catvar) As above, and adjust for clustering with clusters defined by cvar and an intraclass correlation of 0.5 in the two groups ztest v1, by(catvar) cluster(cvar) rho(0.5) Unpaired test of equality of the means of v2 and v3 ztest v2 == v3, unpaired Paired test of equality of the means of v2 and v3 with standard deviation of the differences between paired observations of 2.4 ztest v2 == v3, sddiff(2.4) As above, specified using a common standard deviation of 2 and correlation between observations of 0.28 ztest v2 == v3, sd(2) corr(0.28) -

Analysis of Variance---Why It Is More Important Than Ever
The Annals of Statistics 2005, Vol. 33, No. 1, 1–33 DOI: 10.1214/009053604000001048 c Institute of Mathematical Statistics, 2005 DISCUSSION PAPER ANALYSIS OF VARIANCE—WHY IT IS MORE IMPORTANT THAN EVER1 By Andrew Gelman Columbia University Analysis of variance (ANOVA) is an extremely important method in exploratory and confirmatory data analysis. Unfortunately, in com- plex problems (e.g., split-plot designs), it is not always easy to set up an appropriate ANOVA. We propose a hierarchical analysis that au- tomatically gives the correct ANOVA comparisons even in complex scenarios. The inferences for all means and variances are performed under a model with a separate batch of effects for each row of the ANOVA table. We connect to classical ANOVA by working with finite-sample variance components: fixed and random effects models are character- ized by inferences about existing levels of a factor and new levels, respectively. We also introduce a new graphical display showing in- ferences about the standard deviations of each batch of effects. We illustrate with two examples from our applied data analysis, first illustrating the usefulness of our hierarchical computations and displays, and second showing how the ideas of ANOVA are helpful in understanding a previously fit hierarchical model. 1. Is ANOVA obsolete? What is the analysis of variance? Econometri- cians see it as an uninteresting special case of linear regression. Bayesians see it as an inflexible classical method. Theoretical statisticians have sup- plied many mathematical definitions [see, e.g., Speed (1987)]. Instructors see it as one of the hardest topics in classical statistics to teach, especially arXiv:math/0504499v2 [math.ST] 26 Aug 2005 Received November 2002; revised November 2003. -

Two-Sample Null Hypothesis Significance Tests
City University of New York (CUNY) CUNY Academic Works Open Educational Resources Queensborough Community College 2020 Clear-Sighted Statistics: Module 15: Two-Sample Null Hypothesis Significance estsT Edward Volchok CUNY Queensborough Community College How does access to this work benefit ou?y Let us know! More information about this work at: https://academicworks.cuny.edu/qb_oers/121 Discover additional works at: https://academicworks.cuny.edu This work is made publicly available by the City University of New York (CUNY). Contact: [email protected] Clear-Sighted Statistics: An OER Textbook Module 15: Two-Sample Null Hypothesis Significance Tests Figure 1: The NHST Cycle I. Introduction We covered one-sample hypothesis tests in Module 14. In Module 15, we move on to two- sample hypothesis tests. In doing so, we will review six different statistical significance tests using z-distributions, t-distributions, and F-distributions. After completing this module, you will be able to: • Distinguish between independent and dependent or conditional samples. • Conduct a z-test for two independent means. • Conduct a z-test for two independent proportions. • Conduct a t-test for two independent means assuming equal or pooled variance. • Conduct a t-test for the two independent means assuming unequal variance. • Conduct a F-test for equality of variance in preparation for selecting which two-sample t-tests to use. • Conduct a t-test for the means for two dependent or paired samples. • Measure the size or magnitude of the effect. • Estimate the probability of a Type II error and statistical power for the two-sample z- and t-tests using a power table or G*Power, which is a free program that is widely used by researchers.