Compactum Grown in All Natural Habitats in Morocco
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Initiation Au Maroc
INITIATION AU MAROC INSTITUT DES HAUTES ÉTUDES MAROCAINES INITIATION AU MAROC Nouvelle édition mise à jour VANOEST LES EDITIONS D'ART ET D'HISTOIRE I)i\ R 1 S MCMXLV OAT COLLABORÉ A CET OUVRAGE : 1 MM. É. LÉVI-PROVENÇAL, directeur honoraire de l'Institut des Hautes Études Marocaines, professeur à la Faculté des Lettres d'Alger. J. CÉLÉRIER, directeur d'études de géographie à l'Institut des Hautes Études Marocaines. G. S. COLIN, directeur d'études de linguistique nord-afri- caine à l'Institut des Hautes Études Marocaines, pro- fesseur à l'École des Langues orientales. L EMBERGER, ancien chef du service botanique à l'Institut Scientifique Chérifien, professeur à la Faculté des Sciences de Montpellier. E. LAOUST, directeur d'études honoraire de dialectes berbè- res à l'Institut des Hautes Études Marocaines. Le docteur H.-P.-J. RENAUD, directeur d'études d'histoire des sciences à l'Institut des Hautes Études Marocaines. HENRI TERRASSE, directeur de l'Institut des Hautes Études Marocaines, correspondant de l'Institut. II MM. RENÉ HOFFHERR, maître des requêtes au Conseil d'État, an- cien directeur des Centres d'études juridiques et admi- nistratives \à l'Institut des Hautes Études Marocaines. PAUL MAUCHAUSSÉ, MARCEL BOUSSER et JACQUES MTLLERON, maîtres de conférences aux Centres d'études juridi- ques et administratives. HENRI MAZOYER, contrôleur civil, CLAUDE ECORCHEVILLE, J. PLASSE et JEAN FINES, contrôleurs civils adjoints. J. VALLET, Chef du Bureau de documentation économique au Service du Commerce. AVANT-PROPOS DE LA PREMIÈRE ÉDITION (1932) Jusqu'à une date récente, le Français qui arrivait au Ma- roc recevait un choc : du premier coup, une impression puissante d'étrangeté, de dépaysement le saisissait. -

Greening the Agriculture System: Morocco's Political Failure In
Greening the Agriculture System: Morocco’s Political Failure in Building a Sustainable Model for Development By Jihane Benamar Mentored by Dr. Harry Verhoeven A Thesis Submitted in Partial Fulfilment of the Requirements for the Award of Honors in International Politics, Edmund A. Walsh School of Foreign Service, Georgetown University, Spring 2018. CHAPTER 1: INTRODUCTION ............................................................................................................ 2 • THE MOROCCAN PUZZLE .................................................................................................... 5 • WHY IS AGRICULTURAL DEVELOPMENT IMPORTANT FOR MOROCCO? .............................. 7 • WHY THE PLAN MAROC VERT? .......................................................................................... 8 METHODOLOGY ................................................................................................................... 11 CHAPTER 2: LITERATURE REVIEW ................................................................................................ 13 • A CONCEPTUAL FRAMEWORK FOR “DEVELOPMENT”....................................................... 14 • ROSTOW, STRUCTURAL ADJUSTMENT PROGRAMS (SAPS) & THE OLD DEVELOPMENT DISCOURSE ......................................................................................................................... 19 • THE ROLE OF AGRICULTURE IN DEVELOPMENT .............................................................. 24 • SUSTAINABILITY AND THE DISCOURSE ON DEVELOPMENT & AGRICULTURE ................ -

Cadastre Des Autorisations TPV Page 1 De
Cadastre des autorisations TPV N° N° DATE DE ORIGINE BENEFICIAIRE AUTORISATIO CATEGORIE SERIE ITINERAIRE POINT DEPART POINT DESTINATION DOSSIER SEANCE CT D'AGREMENT N Casablanca - Beni Mellal et retour par Ben Ahmed - Kouribga - Oued Les Héritiers de feu FATHI Mohamed et FATHI Casablanca Beni Mellal 1 V 161 27/04/2006 Transaction 2 A Zem - Boujad Kasbah Tadla Rabia Boujad Casablanca Lundi : Boujaad - Casablanca 1- Oujda - Ahfir - Berkane - Saf Saf - Mellilia Mellilia 2- Oujda - Les Mines de Sidi Sidi Boubker 13 V Les Héritiers de feu MOUMEN Hadj Hmida 902 18/09/2003 Succession 2 A Oujda Boubker Saidia 3- Oujda La plage de Saidia Nador 4- Oujda - Nador 19 V MM. EL IDRISSI Omar et Driss 868 06/07/2005 Transaction 2 et 3 B Casablanca - Souks Casablanca 23 V M. EL HADAD Brahim Ben Mohamed 517 03/07/1974 Succession 2 et 3 A Safi - Souks Safi Mme. Khaddouj Bent Salah 2/24, SALEK Mina 26 V 8/24, et SALEK Jamal Eddine 2/24, EL 55 08/06/1983 Transaction 2 A Casablanca - Settat Casablanca Settat MOUTTAKI Bouchaib et Mustapha 12/24 29 V MM. Les Héritiers de feu EL KAICH Abdelkrim 173 16/02/1988 Succession 3 A Casablanca - Souks Casablanca Fès - Meknès Meknès - Mernissa Meknès - Ghafsai Aouicha Bent Mohamed - LAMBRABET née Fès 30 V 219 27/07/1995 Attribution 2 A Meknès - Sefrou Meknès LABBACI Fatiha et LABBACI Yamina Meknès Meknès - Taza Meknès - Tétouan Meknès - Oujda 31 V M. EL HILALI Abdelahak Ben Mohamed 136 19/09/1972 Attribution A Casablanca - Souks Casablanca 31 V M. -

Plan D'action 2019 Et Programme Prévisionnel 2020-2021
47 Agence urbaine de Taza-Taounate 17 ème conseil d'administration 2017 Plan d'action 2019 et programme prévisionnel 2020-2021 -Plan d’action 2019 Axes Intitulé de l’action Montant Participation en Dh partenaires Homologation Homologation de 10 documents d’urbanisme - - Documents d’urbanisme Plan d’aménagement du centre Bni Mtir– c. Bouhlou- 300.000 50% Pce de Taza Plan d’aménagement du centre Tizi Ouasli- Pce de Taza 300.000 50% Plan d’aménagement du centre Tainaste- Pce de Taza 300.000 50% Plan d’aménagement du centre EL Bssabssa- Pce de 300.000 50% Taounate Plan d’aménagement du centre Bni Oulid -Pce de 300.000 50% Taounate Lancement ou Plan d’aménagement du centre khlalfa -Pce de 300.000 50% actualisation Taounate Plan de développement de l’agglomération rurale El 300.000 50% Gouzate Pce de Taza. Plan de développement de l’agglomération rurale El 200.000 oulja Pce de taounate. Développement rural Mise en œuvre du programme d’assistance architecturale en milieu rural 300.000 Etude d’ordonnancement architectural des axes structurants des centres de 700.000 la province de taounate( Bni oulid, Ain Aicha, Galaz, Mezraoua, Ouartzagh, Kissane, Tafrant, Ain Madiouna, et Khlalfa) Redressement Lancement de 23 plans de redressement et achèvement de 07 quartiers En interne urbanistique lancés avant 2019 Support Prise de vue aérienne et restitution de la ville de Tahla (1/1000) et des centres 600.000 - cartographique de Maghraoua, Fennassa et Moulay Bouchta sur une superficie de 8 000 Ha Total 3.900.000 49 Agence urbaine de Taza-Taounate 17 ème -

Deliverable 1
Lot No. 4 : Project Final Evaluation : « Financial services », Agency for Partnership for Progress – MCA ‐ Morocco Contract No. APP/2012/PP10/QCBS/ME‐16‐lot 4 Deliverable 1: Methodology Report Submitted by : North South Consultants Exchange JUNE 19TH 2013 TABLE OF CONTENTS 1.INTRODUCTION ............................................................................................................................ 1 1.1.CONTEXT ................................................................................................................................................... 1 1.2.OVERVIEW OF THE FINANCIAL SERVICES PROJECT ..................................................................................... 2 1.3.PURPOSE OF THE FSP FINAL EVALUATION ............................................................................................. 4 2.METHODOLOGY ......................................................................................................................................... 5 2.1. COMPREHENSIVE APPROACH .......................................................................................................... 5 2.2. STAKEHOLDERS .......................................................................................................................... 6 2.2.1. APP ................................................................................................................................................... 6 2.2.2. Supervisory Institution ..................................................................................................... -

Etat Global PRDTS CONSEIL REGIONAL 2016
Région Fès-Meknès Direction Générale des Services Division d'Equipement Rural ETAT D'AVANCEMENT DES PROJETS PRDTS/CONSEIL REGIONAL FES-MEKNES/2016 ETAT GLOBAL Contribution Crédits Taux Préfécture / Crédits Commune Intitulé du projet secteur maitre d'ouvrage de la Région engagés Statut avancement avancement Observations Province émis (en KDH) (Kdh) (kDH) physique (en %) Fritissa, Tissaf Ermila, El Orjane, Ouled Ali versement effectué:12\05\2017 Boulemane Youssef,Missour, Mise à niveau de classes scolaires EDUCATION AREF 10 600,00 10 600,00 10 600,00 Achevé 100% Travaux achevés Outat El Haj, Enjil,Sidi Boutayeb, El Mers et Ait Baza DRETL/FES- versement effectué:30\12\2017 Boulemane Enjil Réhabilitation de la RR 503(PK 125 au PK 147) ROUTES/PISTES 21 600,00 21 600,00 21 600,00 Achevé 100% MEKNES Travaux achevés Aménagement de la RP 7039 reliant la RR 402 et DRETL/FES- ElHajeb ait bourzouine ROUTES/PISTES 2 000,00 la RP 7070 sur une longueur de 3 Km MEKNES Aménagement de la RP 7007 reliant la RR701 et DRETL/FES- ElHajeb jahjouh le centre de Jahjouh sur une longueur de 11,5 ROUTES/PISTES 4 000,00 6 200,00 4 648,36 Achevé 100% Travaux achevé MEKNES Km Construction de la RP 7057 reliant la ville d'El DRETL/FES- ElHajeb Iqaddar ROUTES/PISTES 1 800,00 Hajeb et la RR 712 sur une longueur de 4,5 Km MEKNES Travaux d'aménagement de la piste DayetSder reliant la RP 716 et douar Ait LahcenOumoussa, Conseil/Région travaux receptionés en date du ElHajeb Bitit ROUTES/PISTES 714,892 588,66 Achevé 100% DayetSder, Maamel Doum, et Ecole DayetSder Fès Meknès -

Pauvrete, Developpement Humain
ROYAUME DU MAROC HAUT COMMISSARIAT AU PLAN PAUVRETE, DEVELOPPEMENT HUMAIN ET DEVELOPPEMENT SOCIAL AU MAROC Données cartographiques et statistiques Septembre 2004 Remerciements La présente cartographie de la pauvreté, du développement humain et du développement social est le résultat d’un travail d’équipe. Elle a été élaborée par un groupe de spécialistes du Haut Commissariat au Plan (Observatoire des conditions de vie de la population), formé de Mme Ikira D . (Statisticienne) et MM. Douidich M. (Statisticien-économiste), Ezzrari J. (Economiste), Nekrache H. (Statisticien- démographe) et Soudi K. (Statisticien-démographe). Qu’ils en soient vivement remerciés. Mes remerciements vont aussi à MM. Benkasmi M. et Teto A. d’avoir participé aux travaux préparatoires de cette étude, et à Mr Peter Lanjouw, fondateur de la cartographie de la pauvreté, d’avoir été en contact permanent avec l’ensemble de ces spécialistes. SOMMAIRE Ahmed LAHLIMI ALAMI Haut Commissaire au Plan 2 SOMMAIRE Page Partie I : PRESENTATION GENERALE I. Approche de la pauvreté, de la vulnérabilité et de l’inégalité 1.1. Concepts et mesures 1.2. Indicateurs de la pauvreté et de la vulnérabilité au Maroc II. Objectifs et consistance des indices communaux de développement humain et de développement social 2.1. Objectifs 2.2. Consistance et mesure de l’indice communal de développement humain 2.3. Consistance et mesure de l’indice communal de développement social III. Cartographie de la pauvreté, du développement humain et du développement social IV. Niveaux et évolution de la pauvreté, du développement humain et du développement social 4.1. Niveaux et évolution de la pauvreté 4.2. -
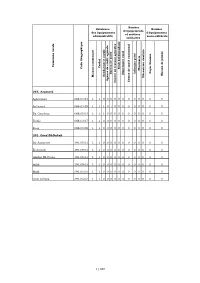
M a Is O N C O M M U N a Le C a Ïd a T G E N D a Rm E Rie Ro Y a Le A
Nombre Existence Nombre d'équipements des équipements d'équipements et services administratifs socio-culturels sanitaires Commune rurale Caïdat Code Géographique Souk Souk hebdomadaire Pharmacie Foyer féminin Infirmier privé Bureau deBureau poste Maison de jeunes Dispensaire rural Maison communale Mécanicien dentiste Gendarmerie royale Agence de crédit agricole Centre de santé communal Centre de travaux agricoles 066. Aousserd Aghouinite 066.03.03 1 1 0 0 0 0 0 0 0 0 0 0 0 0 Aousserd 066.03.05 1 1 1 0 1 0 0 0 0 0 0 0 0 0 Bir Gandouz 066.05.03 1 1 1 0 0 0 0 0 0 0 0 0 0 0 Tichla 066.03.07 1 1 0 0 0 0 0 0 0 0 0 0 0 0 Zoug 066.03.09 1 1 0 0 0 0 0 0 0 0 0 0 0 0 391. Oued Ed-Dahab Bir Anzarane 391.05.01 1 1 0 0 0 0 0 0 0 0 0 0 0 0 El Argoub 391.09.01 1 1 0 0 0 0 0 0 0 0 0 0 0 0 Gleibat EL Foula 391.05.03 1 1 0 0 0 0 0 0 0 0 0 0 0 0 Imlili 391.09.03 1 1 0 0 0 0 0 0 0 0 0 0 0 0 Mijik 391.05.05 1 1 0 0 0 0 0 0 0 0 0 0 0 0 Oum Dreyga 391.05.07 1 1 0 0 0 0 0 0 0 0 0 0 0 0 1/160 La commune Nombre d'établissements est Réseaux d'enseignement et de accessible d'infrastructure formation par Commune rurale Train Lycée Collège Autocar de développement ? Grand taxi Autre moyen Réseau d'électricité Réseau d'eau potable Ecole primaire satellite Ecole primaire centrale professionnelle publique Ecole coranique ou Msid Réseau d'assainissement La commune dispose-t-elle d'un plan Ecole primaire autonome Etablissement de formation 066. -

Télécharger Le Document
CARTOGRAPHIE DU DÉVELOPPEMENT LOCAL MULTIDIMENSIONNEL NIVEAU ET DÉFICITS www.ondh.ma SOMMAIRE Résumé 6 Présentation 7 1. Approche méthodologique 8 1.1. Portée et lecture de l’IDLM 8 1.2. Fiabilité de l’IDLM 9 2. Développement, niveaux et sources de déficit 10 2.1. Cartographie du développement régional 11 2.2. Cartographie du développement provincial 13 2.3. Développement communal, état de lieux et disparité 16 3. L’IDLM, un outil de ciblage des programmes sociaux 19 3.1 Causes du déficit en développement, l’éducation et le niveau de vie en tête 20 3.2. Profil des communes à développement local faible 24 Conclusion 26 Annexes 27 Annexe 1 : Fiabilité de l’indice de développement local multidimensionnel (IDLM) 29 Annexe 2 : Consistance et méthode de calcul de l’indice de développement local 30 multidimensionnel Annexe 3 : Cartographie des niveaux de développement local 35 Annexes Communal 38 Cartographie du développement communal-2014 41 5 RÉSUMÉ La résorption ciblée des déficits socio-économiques à l’échelle locale (province et commune) requiert, à l’instar de l’intégration et la cohésion des territoires, le recours à une cartographie du développement au sens multidimensionnel du terme, conjuguée à celle des causes structurelles de son éventuel retard. Cette étude livre à cet effet une cartographie communale du développement et de ses sources assimilées à l’éducation, la santé, le niveau de vie, l’activité économique, l’habitat et les services sociaux, à partir de la base de données «Indicateurs du RGPH 2014» (HCP, 2017). Cette cartographie du développement et de ses dimensions montre clairement que : - La pauvreté matérielle voire monétaire est certes associée au développement humain, mais elle ne permet pas, à elle seule, d’identifier les communes sous l’emprise d’autres facettes de pauvreté. -

Province Taza FR A3
N MOULAY AHMED CHERIF SIDI BOUTMIM 2 P MIDAR P4102 TARGUIST 52 ZAOUIAT SIDI ABDELKADER ARBAA TAOURIRT N2 07 4 ROYAUME DU MAROC P520 MINISTRE DE L'EQUIPEMENT DU TRANSPORT, DE LA LOGISTIQUE ET DE L'EAU ISSAGUEN N R ZARKAT 2 5 N2 1 1 KETAMA 4 0 9 2 0 R RESEAU ROUTIER 5 5 R P 5 0 P 5 BNI BOUNSAR ADOUZN8 5204 Province : Taza P5400 5 1 SIDI BOUZINEB N TAMASSAOUT BNI BCHIR SIDI ALI BOURAKBA RATBA P TAGHZOUT 00 53 5 TIZI OUASLI P 3 1 1 0 3 ABDELGHAYA SOUAHEL 2 2 5 9 0 3 3 P 5 3 BAB MANSOURA P 5 5 BOURD R510 0 P 5 3 02 OUDKA R 1 53 3 P 5 N 8 5402 P BNI BOUCHIBET P 304 P5 7 TAFRAOUTE 5 3 31 3 P5 5 P R51 R 0 5 KHLALFAN8 0 0 1 8 510 5 R R AKNOUL N THAR ES-SOUK 8 P5 P 510 0 3 8 06 5 R 5 3 P5413 P5404 R 2 P5413 3 RHAFSAIP 5322/05/2018 5 08 0 5 SAKA 8.54.25 0 8.5 R R 1 4 MELLAL N 19 1 3 KM P5 P531 7 1 3 2 2 5 SIDI MOKHFI 10 BOUHOUDA 5 P ZRIZER 3 P 5 5 4 Réalisation : R51 P 0 8 0 BNI OULID 6 Centre NationalR d'Etudes et de Recherches Routières N 4 P P 13 Sidi Bouchetta 1 5 3 5 P54 9 7 3 © CNER / Copie et Reproduction interdite 1 10 R512 3 2 TAINASTE OURTZAGH 5 5 4 Ajbama www.cner.ma P 31 R GALAZ TAOUNATE P5 8 4 0 0 Ü JBARNA 4 8 R P Cherfat Ankad 5 N 4 7 R5 5 P 1 0 1 5 8 0 1 R408 8 3 5 3 EL GOUZATE 5 3 1 KAF EL GHAR P540 R 5 N AIN MEDIOUNA8 4 BNI FTAH R508 MAZGUITAN P P5322 P R508 08 R408 5 5 5 R R 4 314 2 0 P5 5 1 5 404 0 P5 R 8 P AIN EL MA P5 5 4 4 8 12 0 N P 8 P P5404 5 P 5 4 1 5 OULAD3 DAOUD BRARHA 2 1 3 2 3 1 0 AIN MAATOUF 5 TAIFA 0 5 9 9 N 1 R P 0 0 8 5 HAD MSILA 4 4 5 3 5 JBABRA 09 P R 5 3 Sidi Slimane Chraa P P5328 3 Al Azira TRAIBA 3 R BAB EL -

Ethnobotanical Uses and Distribution Status of Arbutus Unedo in Morocco Faida Rahima, Aabdousse Jamal, Boulli Abdelali, Bouda Said, Wahid Nadya
Ethnobotanical uses and distribution status of Arbutus unedo in Morocco Faida Rahima, Aabdousse Jamal, Boulli Abdelali, Bouda Said, Wahid Nadya Databases and Inventories Résumé Contexte: L’arbousier est un arbre fruitier à haute Abstract valeur, environnementale, économique et médicale, Background: Arbutus is a fruit tree with high en raison de ces propriétés attribuées aux ornamental, environmental, economic and medical différentes parties biologiques. Compte tenu de cet value, because of the properties attributed to intérêt, la présente étude s'intéresse à la description different biological parts. Given this interest, the de la répartition, écologie et du statut present study is interested in describing the ethnobotanique des populations naturelles de cette distribution, ecology and ethnobotanical status of espèce. natural populations. Méthodes: L'aire de répartition de cette espèce a été Methods: The range of this species has been largely largement prospectée et explorée afin de localiser prospected and explored to locate their natural ses populations naturelles et de décrire leur populations and describe their distribution and répartition et leur conservation. Pour les utilisations conservation. For ethnobotanical uses of Arbutus in ethnobotaniques d’Arbutus au Maroc, nous avons Morocco, we compiled available data from literature. rassemblé les données disponibles provenant de la littérature. Results: The prospection of the Arbutus natural distribution areas in Morocco has shown that the Correspondence species is growing in different biogeographical regions. Moreover, of its ecological plasticity and Faida Rahima1,2, Aabdousse Jamal 1, Boulli phyto-association, its resistance to mutilations and Abdelali1, Bouda Said2, Wahid Nadya1* its great dynamism, it is very present in the everyday uses of foresters, farmers and rural populations. -

MOROCCO Cannabis Survey 2004
ROYAUME DU MAROC LE PREMIER MINISTRE AGENCE POUR LA PROMOTION ET LE DEVELOPPEMENT ECONOMIQUE ET SOCIAL DES PREFECTURES ET PROVINCES DU NORD DU ROYAUME Vienna International Centre, P.O. Box 500, A-1400 Vienna, Austria Tel: (+43 1) 26060-0, Fax: (+43 1) 26060-5866, www.unodc.org MOROCCO Cannabis Survey 2004 Executive Summary 2004 May 2005 Abbreviations APDN Agence pour la Promotion et le Développement Economique et Social des Préfectures et Provinces du Nord du Royaume CRTS Centre Royal de Télédétection spatiale DPAE Direction de la Programmation et des Affaires Economiques du Ministère de l’Agriculture, du Développement Rural et des Eaux et Forêts Dh Moroccan Dirham GPS Global Positioning System ICMP Illicit Crop Monitoring Programme LARATES Laboratoire de Recherches et d’Analyses Techniques et Scientifiques, Gendarmerie Royale UNODC United Nations Office on Drugs and Crime $ United States dollars Acknowledgments The following institutions and individuals contributed to the implementation of this survey and to the preparation of the present report: Government of Morocco: APDN : Direction des Affaires Administratives et Financières Direction des Opérations Département de la Coopération Internationale Département des Secteurs Productifs CRTS Centre Royal de Télédétection spatiale DPAE : Division des Statistiques et de l’Informatique LARATES : Département Analyses et Prélèvements, Gendarmerie Royale Association TARGA The implementation of this survey would not have been possible without the support of the local administration of the prefectures