Article ISSN 1175-5334 (Online Edition) Urn:Lsid:Zoobank.Org:Pub:9FC2166A-A0B8-4844-9EF6-8E325AD8F531
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Article ISSN 1175-5334 (Online Edition) Urn:Lsid:Zoobank.Org:Pub:9FC2166A-A0B8-4844-9EF6-8E325AD8F531
Zootaxa 3498: 45–62 (2012) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ ZOOTAXA Copyright © 2012 · Magnolia Press Article ISSN 1175-5334 (online edition) urn:lsid:zoobank.org:pub:9FC2166A-A0B8-4844-9EF6-8E325AD8F531 A new species of the genus Oligodon Fitzinger, 1826 (Squamata: Colubridae) from northern Vietnam, southern China and central Laos PATRICK DAVID1, TRUONG QUANG NGUYEN2, TAO THIEN NGUYEN3, KE JIANG4, TIANBO CHEN5, ALEXANDRE TEYNIÉ6 & THOMAS ZIEGLER7 1Reptiles & Amphibiens, UMR 7205 OSEB, Département Systématique et Évolution, CP 30, Muséum National d’Histoire Naturelle, 57 rue Cuvier, 75231 Paris Cedex 05, France. E-mail: [email protected] 2Institute of Ecology and Biological Resources, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet Road, Hanoi, Vietnam. E-mail: [email protected] Present address: Department of Terrestrial Ecology, Cologne Biocenter, University of Cologne, Zülpicher Strasse 47b, D-50674 Cologne, Germany. 3Vietnam National Museum of Nature, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet Road, Hanoi, Vietnam. E-mail: [email protected] 4State Key Laboratory of Genetic Resources and Evolution and Yunnan Laboratory of Molecular Biology of Domestic Animals, Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming, Yunnan, People’s Republic of China. E-mail: [email protected] 5Management Bureau of Nonggang National Nature Reserve, Longzhou 532400, Guangxi A. R., China. E-mail: [email protected] 6Société d’Histoire Naturelle Alcide d’Orbigny, 57, rue de Gergovie, 63170 Aubière, France. E-mail: [email protected] 7Cologne Zoo, Riehler Str. 173, D-50735 Köln, Germany. E-mail: [email protected] Abstract A new species of the genus Oligodon Fitzinger, 1826, Oligodon nagao sp. -
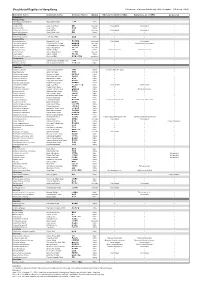
Checklist of Reptiles in Hong Kong © Programme of Ecology & Biodiversity, HKU (Last Update: 10 September 2012)
Checklist of Reptiles in Hong Kong © Programme of Ecology & Biodiversity, HKU (Last Update: 10 September 2012) Scientific name Common name Chinese Name Status Ades & Kendrick (2004) Karsen et al. (1998) Uetz et al. Testudines Platysternidae Platysternon megacephalum Big-headed Terrapin 大頭龜 Native Cheloniidae Caretta caretta Loggerhead Turtle 蠵龜 Uncertain Not included Not included Chelonia mydas Green Turtle 緣海龜 Native Eretmochelys imbricata Hawksbill Turtle 玳瑁 Uncertain Not included Not included Lepidochelys olivacea Pacific Ridley Turtle 麗龜 Native Dermochelyidae Dermochelys coriacea Leatherback Turtle 稜皮龜 Native Geoemydidae Cuora amboinensis Malayan Box Turtle 馬來閉殼龜 Introduced Not included Not included Cuora flavomarginata Yellow-lined Box Terrapin 黃緣閉殼龜 Uncertain Cistoclemmys flavomarginata Cuora trifasciata Three-banded Box Terrapin 三線閉殼龜 Native Mauremys mutica Chinese Pond Turtle 黃喉水龜 Uncertain Mauremys reevesii Reeves' Terrapin 烏龜 Native Chinemys reevesii Chinemys reevesii Ocadia sinensis Chinese Striped Turtle 中華花龜 Uncertain Sacalia bealei Beale's Terrapin 眼斑水龜 Native Trachemys scripta elegans Red-eared Slider 巴西龜 / 紅耳龜 Introduced Trionychidae Palea steindachneri Wattle-necked Soft-shelled Turtle 山瑞鱉 Uncertain Pelodiscus sinensis Chinese Soft-shelled Turtle 中華鱉 / 水魚 Native Squamata - Serpentes Colubridae Achalinus rufescens Rufous Burrowing Snake 棕脊蛇 Native Achalinus refescens (typo) Ahaetulla prasina Jade Vine Snake 綠瘦蛇 Uncertain Amphiesma atemporale Mountain Keelback 無顳鱗游蛇 Native -

List of Reptile Species in Hong Kong
List of Reptile Species in Hong Kong Family No. of Species Common Name Scientific Name Order TESTUDOFORMES Cheloniidae 4 Loggerhead Turtle Caretta caretta Green Turtle Chelonia mydas Hawksbill Turtle Eretmochelys imbricata Olive Ridley Turtle Lepidochelys olivacea Dermochelyidae 1 Leatherback Turtle Dermochelys coriacea Emydidae 1 Red-eared Slider * Trachemys scripta elegans Geoemydidae 3 Three-banded Box Turtle Cuora trifasciata Reeves' Turtle Mauremys reevesii Beale's Turtle Sacalia bealei Platysternidae 1 Big-headed Turtle Platysternon megacephalum Trionychidae 1 Chinese Soft-shelled Turtle Pelodiscus sinensis Order SQUAMATA Suborder LACERTILIA Agamidae 1 Changeable Lizard Calotes versicolor Lacertidae 1 Grass Lizard Takydromus sexlineatus ocellatus Scincidae 11 Chinese Forest Skink Ateuchosaurus chinensis Long-tailed Skink Eutropis longicaudata Chinese Skink Plestiodon chinensis chinensis Five-striped Blue-tailed Skink Plestiodon elegans Blue-tailed Skink Plestiodon quadrilineatus Vietnamese Five-lined Skink Plestiodon tamdaoensis Slender Forest Skink Scincella modesta Reeve's Smooth Skink Scincella reevesii Brown Forest Skink Sphenomorphus incognitus Indian Forest Skink Sphenomorphus indicus Chinese Waterside Skink Tropidophorus sinicus Varanidae 1 Common Water Monitor Varanus salvator Dibamidae 1 Bogadek's Burrowing Lizard Dibamus bogadeki Gekkonidae 8 Four-clawed Gecko Gehyra mutilata Chinese Gecko Gekko chinensis Tokay Gecko Gekko gecko Bowring's Gecko Hemidactylus bowringii Brook's Gecko* Hemidactylus brookii House Gecko* Hemidactylus -

Hue University College of Education Do Trong Dang
HUE UNIVERSITY COLLEGE OF EDUCATION DO TRONG DANG RESEARCH ON THE SPECIES DIVERSITY, DISTRIBUTION AND CONSERVATION VALUE OF AMPHIBIANS AND REPTILES SPECIES IN SOUTH PART OF CU MONG PASS, PHU YEN PROVINCE Major: Zoology Code: 62 42 01 03 SUMMARY OF Ph.D IN BIOLOGY Instructors PhD. Nguyen Quang Truong Prof. PhD. Ngo Dac Chung HUE, 2017 The work was completed in: The work was completed in: College of Education, Hue University Science instructors: PhD. Nguyen Quang Truong Prof. PhD. Ngo Dac Chung Reviewer 1: Reviewer 2: Reviewer 3: The thesis was defended at the Council of thesis assessment of Hue University Council held at: 4 Le Loi street, Hue city, Thua Thien Hue province, at ……………………………………... on .…../…../2017 Theses can be further referred at: 1. National Library 2. Center for Information and Library of College of Education, Hue University WORKS RELATED TO THE THESIS HAS BEEN PUBLISHED 1. Dang Trong Do, Chung Dac Ngo, Truong Quang Nguyen (2015), Diversity of the narrow-mouth frogs (Amphibia: Anura: Microhylidae) from Phu Yen Province, In Proceedings of the sixth National Scientific Conference on Ecology and Biological Resources, pp. 515-519. 2. Dang Trong Do, Chung Dac Ngo, Truong Quang Nguyen (2016), New records of turtles from Phu Yen Province, Vietnam, In Proceedings of the 2ND National Scientific Conference on Biologycal Research and Teaching in Vietnam, pp. 129-136. 3. Dang Trong Do, Chung Dac Ngo, Truong Quang Nguyen (2016), New records of Colubridae (Squamata: Serpentes) and an updated list of snakes from Phu Yen Province, Vietnam, In Proceedings of third National Scientific Conference on Amphibians and Reptiles in Vietnam, pp. -
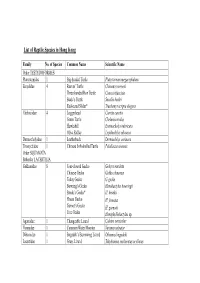
List of Reptile Species in Hong Kong
List of Reptile Species in Hong Kong Family No. of Species Common Name Scientific Name Order TESTUDOFORMES Platysternidae 1 Big-headed Turtle Platysternum megacephalum Emydidae 4 Reeves’ Turtle Chinemys reevesii Three-banded Box Turtle Cuora trifasciata Beale’s Turtle Sacalia bealei Red-eared Slider* Trachemys scripta elegans Cheloniidae 4 Loggerhead Caretta caretta Green Turtle Chelonia mydas Hawksbill Eretmochelys imbricata Olive Ridley Lepidochelys olivacea Dermochelyidae 1 Leatherback Dermochelys coriacea Trionychidae 1 Chinese Soft-shelled Turtle Pelodiscus sinensis Order SQUAMATA Suborder LACERTILIA Gekkonidae 8 Four-clawed Gecko Gehyra mutilata Chinese Gecko Gekko chinensis Tokay Gecko G. gecko Bowring’s Gecko Hemidactylus bowringii Brook’s Gecko* H. brookii House Gecko H. frenatus Garnot’s Gecko H. garnotii Tree Gecko Hemiphyllodactylus sp. Agamidae 1 Changeable Lizard Calotes versicolor Varanidae 1 Common Water Monitor Varanus salvator Dibamidae 1 Bogadek’s Burrowing Lizard Dibamus bogadeki Lacertidae 1 Grass Lizard Takydromus sexlineatus ocellatus Scincidae 11 Chinese Forest Skink Ateuchosaurus chinensis Chinese Skink Eumeces chinensis chinensis Five-striped Blue-tailed Skink E. elegans Blue-tailed Skink E. quadrilineatus Vietnamese Five-lined Skink E. tamdaoensis Long-tailed Skink Mabuya longicaudata Slender Forest Skink Scincella modesta Reeve’s Smooth Skink S. reevesii Indian Forest Skink Sphenomorphus indicus Brown Forest Skink S. incognitus Chinese Waterside Skink Tropidophorus sinicus Order SQUAMATA Suborder SERPENTES Typhlopidae 3 Lazell’s Blind Snake Typhlops lazelli White-headed Blind Snake Ramphotyphlops albiceps Common Blind Snake R. braminus Boidae 1 Burmese Python Python molurus bivittatus Colubridae 34 Rufous Burrowing Snake Achalinus rufescens Jade Vine Snake Ahaetulla prasina medioxima Mountain Keelback Amphiesma atemporale White-browed Keelback A. boulengeri Buff-striped Keelback A. -

Human-Snake Conflict Patterns in a Dense Urban-Forest Mosaic Landscape
Herpetological Conservation and Biology 14(1):143–154. Submitted: 9 June 2018; Accepted: 25 February 2019; Published: 30 April 2019. HUMAN-SNAKE CONFLICT PATTERNS IN A DENSE URBAN-FOREST MOSAIC LANDSCAPE SAM YUE1,3, TIMOTHY C. BONEBRAKE1, AND LUKE GIBSON2 1School of Biological Sciences, University of Hong Kong, Pokfulam, Hong Kong, China 2School of Environmental Science and Engineering, Southern University of Science and Technology, Shenzhen, China 3Corresponding author, e-mail: [email protected] Abstract.—Human expansion and urbanization have caused an escalation in human-wildlife conflicts worldwide. Of particular concern are human-snake conflicts (HSC), which result in over five million reported cases of snakebite annually and significant medical costs. There is an urgent need to understand HSC to mitigate such incidents, especially in Asia, which holds the highest HSC frequency in the world and the highest projected urbanization rate, though knowledge of HSC patterns is currently lacking. Here, we examined the relationships between season, weather, and habitat type on HSC incidents since 2002 in Hong Kong, China, which contains a mixed landscape of forest, dense urban areas, and habitats across a range of human disturbance and forest succession. HSC frequency peaked in the autumn and spring, likely due to increased activity before and after winter brumation. There were no considerable differences between incidents involving venomous and non-venomous species. Dense urban areas had low HSC, likely due to its inhospitable environment for snakes, while forest cover had no discernible influence on HSC. We found that disturbed or lower quality habitats such as shrubland or areas with minimal vegetation had the highest HSC, likely because such areas contain intermediate densities of snakes and humans, and intermediate levels of disturbance. -

A New Species of Kukri Snake (Squamata: Colubridae: Oligodon Fitzinger, 1826) from Con Dao Islands, Southern Vietnam
Zootaxa 4139 (2): 261–273 ISSN 1175-5326 (print edition) http://www.mapress.com/j/zt/ Article ZOOTAXA Copyright © 2016 Magnolia Press ISSN 1175-5334 (online edition) http://doi.org/10.11646/zootaxa.4139.2.9 http://zoobank.org/urn:lsid:zoobank.org:pub:EFC1515F-C332-40E4-A352-EA89F3DC06F7 A new species of kukri snake (Squamata: Colubridae: Oligodon Fitzinger, 1826) from Con Dao Islands, southern Vietnam SANG NGOC NGUYEN1,4, VU DANG HOANG NGUYEN1, SON HONG LE2 & ROBERT W. MURPHY3 1Institute of Tropical Biology, Vietnam Academy of Science and Technology, 85 Tran Quoc Toan St., Dist. 3, Ho Chi Minh City, Viet- nam. E-mail: [email protected] 2Con Dao National Park, 29 Vo Thi Sau St., Con Dao Town, Ba Ria–Vung Tau Prov., Vietnam. E-mail: [email protected] 3Centre for Biodiversity and Conservation Biology, Royal Ontario Museum, 100 Queen’s Park, Toronto, Canada M5S 2C6. E-mail: [email protected] 4Corresponding author Abstract We describe a new kukri snake, Oligodon condaoensis sp. nov., from Con Dao Islands, southern Vietnam based on the morphological characters of four specimens. It differs from other congeners by a combination of the following characters: medium size in adults (total length up to 552 mm); 17–17–15 dorsal scale rows; deeply forked hemipenes without spines and papillae, extending to subcaudal 13 or 14; 11–13 maxillary teeth, the posterior three being enlarged; cloacal plate un- divided; head scalation complete; nasal divided; presubocular absent; 168–176 ventrals; 33–37 subcaudals; overall dorsal coloration dark gray, faint body stripes present or absent; and ventral coloration cream to dark gray without rectangular blotches. -

Ecography ECOG-04374 Chen, C., Qu, Y., Zhou, X
Ecography ECOG-04374 Chen, C., Qu, Y., Zhou, X. and Wang, Y. 2019. Human overexploitation and extinction risk correlates of Chinese snakes. – Ecography doi: 10.1111/ecog.04374 Supplementary material 1 Human overexploitation and extinction risk correlates of Chinese snakes 2 3 Supporting information 4 Appendix 1. Properties of the datasets used, hypotheses and justification 5 Table A1. Extinction risk and predictor variables of Chinese snakes 6 Table A2. Hypotheses on the relationship between extinction risk and intrinsic and extrinsic factors 7 Table A3. Main sources for assessing elevational range and human exploitation index 8 Table A4. The correlation matrices of predictor variables for each snake group 9 Table a5. The full AICc models for Chinese snakes 10 Table a6. The interactions between geographic range size and other important variables 11 Appendix 2. Patterns of extinction risk using the IUCN Red List criteria 12 Appendix 3. Distribution of extinction risk among snake genera 13 Appendix 4. Correlates of extinction risk when species classified under range-based criteria were excluded 14 15 16 17 Appendix 1. Properties of the datasets used, hypotheses and justification 18 Table A1. Extinction risk (based on China Biodiversity Red List), intrinsic traits and extrinsic factors of Chinese snakes. Abbreviations: China, 19 China Biodiversity Red List; RangeC, species assessed under range-based criteria; IUCN, IUCN Red List; BL, Body length; BR, Body ratio; 20 AP, Activity period; MH, Microhabitat; RM, Reproductive mode; HS, Habitat -

Updated Checklist of Indian Reptiles R
Updated Checklist of Indian Reptiles R. Aengals, V.M. Sathish Kumar & Muhamed Jafer Palot* Southern Regional Centre, Zoological Survey of India, Chennai-600 028 *Western Ghat Regional Centre, Zoological Survey of India, Calicut-673 006 Corresponding author: [email protected] INTRODUCTION Reptiles are cold-blooded animals found in almost all the parts of the world, except the very cold regions. In India, all the three living orders of reptiles have their representatives - Crocodylia (crocodiles), Testudines (turtles and tortoises) and Squamata (lizards and snakes). The diversified climate, varying vegetation and different types of soil in the country form a wide range of biotopes that support a highly diversified reptilian fauna. The Western Ghats, Eastern Himalaya, and the Andaman and Nicobar Islands are endowed with varied and unique reptilian fauna. The monumental works on Indian reptiles are, ‘The Reptiles of British India’ by Gunther (1864), ‘Fauna of British India - ‘Reptilia and Batrachia’ by Boulenger (1890) and Smith (1931, 1935, 1943). The work of Smith stood the test of time and forms the standard work on the subject. Further contributions were made by Tiwari & Biswas (1973), Sharma (1977, 1978, 1981, 1998, 2002, 2007), Murthy (1985, 1994, 2010), Das (1991, 1994, 1996, 1997, 2003), Tikedar & Sharma (1992), Das & Bauer (2000), Das & Sengupta (2000), Daniel (2002), Whitaker and Captain (2004), Sharma (2007), Thrope et. al. (2007), Mukherjee and Bhupathy (2007), Gower and Winkler (2007), Manamendra-Arachchi et al. (2007), Das and Vijayakumar (2009), Giri (2008), Giri & Bauer (2008), Giri, et al. (2009a), Giri et al.(2009b), Zambre et al. (2009), Haralu (2010), Pook et al.(2009), Van Rooijen and Vogel (2009), Mahony (2009, 2010) and Venugopal (2010). -

CAMP Workshop for All Indian Reptiles (BCPP)
Biodiversity Conservation Prioritisation Project (BCPP) India Endangered Species Project Conservation Assessment and Management Plan (C.A.M.P.) Workshops REPORT By Zoo Outreach Organisation / CBSG, India 1998 Authored by Participants Edited by Sanjay Molur and Sally Walker Published by Zoo Outreach Organisation Reptiles of India Hosted by the Forest Department of Tamil Nadu Coimbatore, 19 – 23 May 1997 Zoo Outreach Organisation/ CBSG, India, 79 Bharati Colony, Peelamedu, Coimbatore 641 004, Tamil Nadu, India CITATION Sanjay Molur P.O. Nameer & Sally Walker (eds.) (1998). Report of the Workshop “Conservation Assessment and Management Plan for Mammals of India” (BCPP- Endangered Species Project), Zoo Outreach Organisation, Conservation Breeding Specialist Group, India, Coimbatore, India. 175 p. Report # 17. (1998) Zoo Outreach Organisation/ Conservation Breeding Specialist Group, India PB 1683, 79, Bharathi Colony, Peelamedu, Coimbatore 641 004, Tamil Nadu, India Ph: 91 (422) 57 10 87; Fax: 91 (422) 57 32 69; e-mail: [email protected] Cover design, typesetting and printing: Zoo Outreach Organisation Contents Reptiles of India Authors of the Report and participating institutions I-ii Sponsors and organisers iii-iv Executive Summary 1-13 Summary Data Tables 15-28 Report 29-69 Taxon Data Sheets 71-175 Acknowledgement Dr. Ajith Kumar, Scientist, Salim Ali Centre for Ornithology and Natural History, was Coordinator of the Endangered Species component of the Biodiversity Conservation Prioritisation Project and, as such, our Advisor and Guide for the workshops. We would like to acknowledge him for suggesting the CAMP process and IUCN Red List Criteria as a means of assessment at an early stage and ZOO/CBSG, India as a possible organiser of the workshops. -

The Status of Herpetofauna of Bhutan
Review Paper The status of herpetofauna of Bhutan 1* Jigme Tshelthrim Wangyal Nanorana sp. from Rigsoom Gonpa, Trashiyangtse Abstract gularis), Annandali’s Paa (Nanorana annandalii), and Pygmy Leaf Frog (Chiromantis This paper presents the state-of-the-knowledge vittatus) bring the total number of species on herpetofauna (reptiles and amphibians) of Bhutan. Through a comprehensive review of reported species, Gharial (Gavialis gangeticus) andknown the in AmericanBhutan to Bull191. FrogTwo (previouslyLithobates catesbeianus) are removed from the list. The oneliterature, caecilian the paperand a identifiesHimalayan 84 Salamander snakes, 23 paper highlights which species need further knownlizards, 20to tortoisesoccur in andBhutan. turtles, Based 56 anurans,on the research or special conservation protection status.confirmation, and which warrant further (author’sOligodon field taeniolatus work, six), previouslyYunnan Bamboo unreported Pit Viperspecies (Trimeresurus of herpetofauna cf. stejnegeri viz. Russel’s yunnanensis Kukri), Keywords: Tibetan Pit Viper (Trimeresurus cf. tibetanus), tortoises and turtles, amphibians, Ptyctolaemus anurans, Bhutan Herpetofauna, snakes, lizards, Blue Fan Throated Lizard ( 1* Corresponding author’s email: [email protected] District Forest Office, District Administration, Trashigang, Bhutan 20 Herpetofauna of Bhutan Introduction the population. As such, they indicate short- term changes in their environment. Therefore, depend on prosthetic devices to keep ourselves andWilson the (1998) biosphere states thatalive, “To thewe extentwill thatrender we Thisstudy paper of the presentstaxa is very a comprehensiveimportant. update on the herpetofauna of Bhutan, to promote the rest of life, we will impoverish our own attention for research priorities in the specieseverything for fragile.all time.” To He the offers extent a that tremendously we banish grave cautionary to Homo sapiens, a caution and the world as a whole. -

From the Cat Tien National Park, Southern Vietnam
Zootaxa 3702 (3): 233–246 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2013 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3702.3.2 http://zoobank.org/urn:lsid:zoobank.org:pub:508E2367-560C-4FC5-9581-8FEB59EFDAC2 A new species of Kukri Snake (Oligodon Fitzinger, 1826; Squamata: Colubridae) from the Cat Tien National Park, southern Vietnam ANNA B. VASSILIEVA1,2,6, PETER GEISSLER5,6, EDUARD A. GALOYAN2,3, NIKOLAY A. POYARKOV Jr1,2, ROBERT WAYNE VAN DEVENDER4 & WOLFGANG BÖHME5 1Department of Vertebrate Zoology, Biological Faculty, Lomonosov Moscow State University, Leninskiye Gory, GSP–1, Moscow 119991, Russia 2Joint Russian–Vietnamese Tropical Research and Technological Center of the A.N. Severtsov Institute of Ecology and Evolution, South Branch, 3, Street 3/2, 10 District, Ho Chi Minh City, Vietnam 3Zoological Museum, Lomonosov Moscow State University, Bolshaya Nikitskaya st. 6., Moscow 125009, Russia 4Department of Biology, Appalachian State University, Boone, NC 28608, USA 5Zoologisches Forschungsmuseum Alexander Koenig, Adenauerallee 160, 53113 Bonn, Germany. E-mail: [email protected] 6Corresponding authors. E-mail: [email protected]; [email protected] Abstract We describe a new species of the genus Oligodon from the lowland forests of Cat Tien National Park, Dong Nai Province, in southern Vietnam. Oligodon cattienensis sp. nov. is distinguished from the remaining Southeast Asian kukri snakes by the combination of the following characters: medium-sized, deeply forked hemipenes without spines, 17-17-15 dorsal scale rows, nasal entire, 2 small postoculars, almost equal in size, 167–178 ventrals, 31–35 subcaudals, 24–35 + 5 large dark-edged vertebral blotches in combination with a yellow-orange or red vertebral stripe between blotches, head pattern including ocular band, temporal bands and elongated chevron, ventrals pink or whitish (reddish in juveniles) in life, some bearing a quadrangular dark blotch on each lateral side, or ventrals being entirely dark.