Moderate Alcohol Consumption in Chronic Form Enhances The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
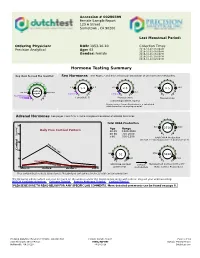
Hormone Testing Summary
Accession # 00280399 Female Sample Report 123 A Street Sometown , CA 90266 Last Menstrual Period: Ordering Physician: DOB: 1953-10-10 Collection Times: Precision Analytical Age: 63 2016-10-02 06:00AM 2016-10-02 08:00AM Gender: Female 2016-10-01 06:00PM 2016-10-01 10:00PM 2016-10-02 02:00AM Hormone Testing Summary Key (how to read the results): Sex Hormones See Pages 2 and 3 for a thorough breakdown of sex hormone metabolites opausa n l R e a m n e g 1.8 4.5 6.0 2.3 14.0 r 5.1 2.8 1.5 P e patient low limit high limit 20.0 result 0.2-0.7 0.3-2.0 Postmenopausal range Estradiol(E2) Progesterone Testosterone (Serum Equivalent, ng/mL) Progesterone Serum Equivalent is a calculated value based on urine pregnanediol. Adrenal Hormones See pages 4 and 5 for a more complete breakdown of adrenal hormones Total DHEA Production 300 500 3000 Age Range 2516 Daily Free Cortisol Pattern 20-39 1300-3000 240 40-60 750-2000 (ng/mg) >60 500-1200 Total DHEA Production (DHEAS + Etiocholanolone + Androsterone) 180 High Range Limit 120Cortisol 80 52 2750 5930 60 230 6500 Patient Values Low Range Limit 24hr Free Cortisol cortisol Metabolized Cortisol (THF+THE) 0 (A+B+C+D) metabolism (Total Cortisol Production) Waking (A) Morning (B) Afternoon (C) Night (D) Free cortisol best reflects tissue levels. Metabolized cortisol best reflects total cortisol production. The following videos (which can also be found on the website under the listed names along with others) may aid your understanding: DUTCH Complete Overview Estrogen Tutorial Female Androgen Tutorial Cortisol Tutorial PLEASE BE SURE TO READ BELOW FOR A NY SPECIFIC LA B COMMENTS. -

The Steroid Metabolome in Men with Mood and Anxiety Disorders
Physiol. Res. 64 (Suppl. 2): S275-S282, 2015 https://doi.org/10.33549/physiolres.933067 The Steroid Metabolome in Men With Mood and Anxiety Disorders M. DUŠKOVÁ1, M. HILL1, M. BIČÍKOVÁ1, M. ŠRÁMKOVÁ1, D. ŘÍPOVÁ2, P. MOHR2, L. STÁRKA1 1Institute of Endocrinology, Prague, Czech Republic, 2National Institute of Mental Health, Klecany, Czech Republic Received May 5, 2015 Accepted May 20, 2015 Summary many other hormones and various factors have been The mood and behavior of individuals result from an orchestra of identified as modulators of mood and behavior in such many factors. Among them steroids play an important role; disorders. Recent studies have described the role of brain- however, only several common hormones have been investigated derived neurotrophic factor (BDNF) (Pluchino et al. in this respect. It has been demonstrated that some steroid 2013, Numakawa et al. 2014), thyroid hormones (Duntas metabolites long considered merely the products of steroid and Mails 2013), inflammation (Halaris 2013), immunity hormone metabolism in fact possess considerable activity in the (Pitychoutis and Papadopoulou-Daifoti 2010), melatonin CNS. For this reason we studied the steroid metabolome (Boyce and Hopwood 2013), oxytocin and vasopressin including 50 analytes in 20 men with depression, 20 men with (Scantamburlo et al. 2009, Matsuzaki et al. 2012), the anxiety and 30 healthy controls. Significant differences were renin-angiotensin-aldosterone system (Franklin et al. found not only between controls and men with either depression 2012, Murck et al. 2012), the cannabinoid system or anxiety, but also between men with depression and anxiety. (Martykánová 2010, Gorzalka and Hill 2011, Smaga et Particularly striking were those steroids until now not generally al. -

Steroid Profiling in Urine of Intact Glucuronidated and Sulfated
CORE Metadata, citation and similar papers at core.ac.uk Provided by Ghent University Academic Bibliography Journal of Chromatography A 1624 (2020) 461231 Contents lists available at ScienceDirect Journal of Chromatography A journal homepage: www.elsevier.com/locate/chroma Steroid profiling in urine of intact glucuronidated and sulfated steroids using liquid chromatography-mass spectrometry ∗ Laurie De Wilde , Kris Roels, Pieter Van Renterghem, Peter Van Eenoo, Koen Deventer 1 Doping Control Laboratory (DoCoLab), Ghent University (UGent), Department Diagnostic Sciences, Technologiepark 30B, B-9052 Zwijnaarde, Belgium a r t i c l e i n f o a b s t r a c t Article history: Detection of endogenous anabolic androgenic steroids (EAAS) misuse is a major challenge in doping con- Received 26 November 2019 trol analysis. Currently, a number of endogenous steroids, which constitute the steroid profile, are quanti- Revised 6 May 2020 fied using gas chromatography (GC). With this methodology, only the sum of the free and glucuronidated Accepted 10 May 2020 steroids is measured together. A dilute-and-shoot LC-MS method, which is compliant with the quality Available online 23 May 2020 requirements for measuring EAAS established by the World Anti-Doping Agency (WADA), was devel- Keywords: oped and validated containing glucuronidated and sulfated steroids in order to gain some extra infor- Doping mation and to expand the existing steroid profile. The developed method is, to the best of our knowl- Urine edge, the first method to combine both steroid glucuronides and sulfates, which is compliant with the Steroid profile quality standards of the technical document on EAAS, established by WADA. -

Increased and Mistimed Sex Hormone Production in Night Shift Workers
Published OnlineFirst March 3, 2015; DOI: 10.1158/1055-9965.EPI-14-1271 Research Article Cancer Epidemiology, Biomarkers Increased and Mistimed Sex Hormone Production & Prevention in Night Shift Workers Kyriaki Papantoniou1,2,3,4, Oscar J. Pozo2, Ana Espinosa1,2,3,4, Josep Marcos2,3, Gemma Castano-Vinyals~ 1,2,3,4, Xavier Basagana~ 1,2,3,4, Elena Juanola Pages 5, Joan Mirabent6,7, Jordi Martín8, Patricia Such Faro9, Amparo Gasco Aparici10, Benita Middleton11, Debra J. Skene11, and Manolis Kogevinas1,2,3,4,12 Abstract Background: Night shift work has been associated with Results: Night workers had higher levels of total progestagens an increased risk for breast and prostate cancer. The effect [geometric mean ratio (GMR) 1.65; 95% confidence intervals of circadian disruption on sex steroid production is a pos- (CI), 1.17–2.32] and androgens (GMR: 1.44; 95% CI, 1.03–2.00), sible underlying mechanism, underinvestigated in hum- compared with day workers, after adjusting for potential con- ans. We have assessed daily rhythms of sex hormones founders. The increased sex hormone levels among night and melatonin in night and day shift workers of both shift workers were not related to the observed suppression of sexes. 6-sulfatoxymelatonin. Peak time of androgens was significantly Methods: We recruited 75 night and 42 day workers, ages later among night workers, compared with day workers (testos- 22 to 64 years, in different working settings. Participants terone: 12:14 hours; 10:06-14:48 vs. 08:35 hours; 06:52-10:46). collected urine samples from all voids over 24 hours on a Conclusions: We found increased levels of progestagens and working day. -

Studies on Steroid Fever II. Pyrogenic and Anti-Pyrogenic Activity in Vitro of Some Endogenous Steroids of Man
Studies on steroid fever II. Pyrogenic and anti-pyrogenic activity in vitro of some endogenous steroids of man G. M. Dillard, Phyllis Bodel J Clin Invest. 1970;49(12):2418-2426. https://doi.org/10.1172/JCI106461. Research Article The pyrogenic properties of some C-19 and C-21 steroids were examined by in vitro incubation of human blood leukocytes with serum-buffer solutions of the steroids and injection of the 18-hr supernatants into rabbits. In previous studies this method demonstrated release of leukocyte endogenous pyrogen by etiocholanolone. With two exceptions, steroids known to cause fever in man, such as 11β-OH etiocholanolone and 3α-hydroxy-5β-pregnane-20-one were also pyrogenic in vitro. All steroids tested which are nonpyrogenic in man, such as androsterone, 3β-OH etiocholanolone, and 3α, 17α-dihydroxy-5β-pregnan-20-one were also nonpyrogenic in vitro. Solubility in aqueous solution did not correlate with pyrogenic capacity. Inhibition of pyrogen release from human leukocytes in vitro by hydrocortisone and estradiol was demonstrated. Hydrocortisone-treated leukocytes released less pyrogen than did normal leukocytes when stimulated either by etiocholanolone or by phagocytosis of heat-killed staphylococci. On the other hand, estradiol-treated blood leukocytes and mononuclear cells showed significant suppression of pyrogen release when phagocytosis, but not etiocholanolone, was used as the stimulus. When blood cells were incubated with progesterone, greater than normal amounts of pyrogen were released following phagocytosis, and the inhibiting effect of estradiol could be partially reversed. Neither estradiol nor hydrocortisone appeared to act on rabbit leukocytes. These studies indicate that a variety of naturally-occurring steroids may alter pyrogen release from leukocytes. -

Development and Application of Methods for Extraction and LC/MS/MS Analysis of Sex Steroids and Conjugates from Fish Feces
Development and Application of Methods for Extraction and LC/MS/MS Analysis of Sex Steroids and Conjugates from Fish Feces By Lisa E. Peters A Thesis submitted to the Faculty of Graduate Studies of The University of Manitoba in partial fulfilment of the requirements of the degree of Doctor of Philosophy Department of Environment and Geography University of Manitoba Winnipeg Copyright © 2014 Lisa E. Peters 1 Acknowledgments I would like to start by thanking my supervisor, Dr. Gregg Tomy, for his support, patience and resources during my Ph.D. program. He took a chance when I tried to convince him that my biology background would be a great addition to his chemistry lab. My husband, Vince Palace, and I also appreciated his support and genuine happiness for us when I announced we were expecting a baby in the middle of my studies. A sincere thanks also goes to my co-supervisor, Dr. Mark Hanson, for his support on so many levels (I wouldn’t even know where to start), and for doing his best to keep me on track, which was no small task. I would also like to thank my other thesis committee members, Drs. Gary Anderson and Feiyue Wang, for their encouragement and insightful comments. Gary gave a lot of extra time to review various thesis chapters and data, and to lend his expertise during the fish surgeries. I also had the pleasure of taking his Endocrinology course, which was probably the most informative and enjoyable class of my entire university student career. I would like to thank the technical staff and students from DFO, Suzanne Mittermuller, Kerry Wautier, Alea Goodmanson, Danielle Godard, Lisa Friedrich and Kirstin Dangerfield, and my lab mates Bruno Rosenberg, Kerri Pleskach, Colin Darling, Bonnie Gemmill and Lianna Bestvater for their technical input and help during my experiments. -

AOD Biennial Review
Introduction The Drug-Free Schools and Communities Act and Part 86 of the Education Department General Administrative Regulations (EDGAR) require each institution of higher education participating in Title IV student financial assistance to certify that it has developed and implemented a program to prevent the unlawful possession, use, and distribution of drugs and alcohol on campus and at institution-recognized events and activities. Annually, the institution must distribute to current students and employees written information about its drug and alcohol abuse prevention program, including information about related sanctions. The distribution plan must include provisions for sharing this information with new students and employees who join the institution at later points in the year as well. In addition, on a biennial basis, the institution must conduct a review to determine the effectiveness of its programs and prepare a report of the review’s findings. The report must be retained and made available to the US Department of Education upon request. Notre Dame of Maryland University (“NDMU” or “the University”) acknowledges these responsibilities, including the timely review of and reporting on the effectiveness of its prevention programs. NDMU is a private, Catholic university established in 1895 with the mission to educate leaders to transform the world. NDMU is a comprehensive university that is home to Maryland's only women's college and offers a wide variety of full and part-time undergraduate, graduate, doctoral, and certificate programs to women and men. The University enrolls approximately 2300 students and has Schools of Arts, Sciences, and Business; Education; Nursing; and Pharmacy. Additionally, the University offers programs for adult students fully online and at several locations throughout the State. -

Alteration of the Steroidogenesis in Boys with Autism Spectrum Disorders
Janšáková et al. Translational Psychiatry (2020) 10:340 https://doi.org/10.1038/s41398-020-01017-8 Translational Psychiatry ARTICLE Open Access Alteration of the steroidogenesis in boys with autism spectrum disorders Katarína Janšáková 1, Martin Hill 2,DianaČelárová1,HanaCelušáková1,GabrielaRepiská1,MarieBičíková2, Ludmila Máčová2 and Daniela Ostatníková1 Abstract The etiology of autism spectrum disorders (ASD) remains unknown, but associations between prenatal hormonal changes and ASD risk were found. The consequences of these changes on the steroidogenesis during a postnatal development are not yet well known. The aim of this study was to analyze the steroid metabolic pathway in prepubertal ASD and neurotypical boys. Plasma samples were collected from 62 prepubertal ASD boys and 24 age and sex-matched controls (CTRL). Eighty-two biomarkers of steroidogenesis were detected using gas-chromatography tandem-mass spectrometry. We observed changes across the whole alternative backdoor pathway of androgens synthesis toward lower level in ASD group. Our data indicate suppressed production of pregnenolone sulfate at augmented activities of CYP17A1 and SULT2A1 and reduced HSD3B2 activity in ASD group which is partly consistent with the results reported in older children, in whom the adrenal zona reticularis significantly influences the steroid levels. Furthermore, we detected the suppressed activity of CYP7B1 enzyme readily metabolizing the precursors of sex hormones on one hand but increased anti-glucocorticoid effect of 7α-hydroxy-DHEA via competition with cortisone for HSD11B1 on the other. The multivariate model found significant correlations between behavioral indices and circulating steroids. From dependent variables, the best correlation was found for the social interaction (28.5%). Observed changes give a space for their utilization as biomarkers while reveal the etiopathogenesis of ASD. -

Androstenedione
NTP TECHNICAL REPORT ON THE TOXICOLOGY ANd CARCINOGENESIS STUdIES OF ANdROSTENEdIONE (CAS NO. 63-05-8) IN F344/N RATS ANd B6C3F1 MICE (GAVAGE STUdIES) NATIONAL TOXICOLOGY PROGRAM P.O. Box 12233 Research Triangle Park, NC 27709 September 2010 NTP TR 560 NIH Publication No. 10-5901 National Institutes of Health Public Health Service U.S. dEPARTMENT OF HEALTH ANd HUMAN SERVICES FOREWORd The National Toxicology Program (NTP) is an interagency program within the Public Health Service (PHS) of the Department of Health and Human Services (HHS) and is headquartered at the National Institute of Environmental Health Sciences of the National Institutes of Health (NIEHS/NIH). Three agencies contribute resources to the program: NIEHS/NIH, the National Institute for Occupational Safety and Health of the Centers for Disease Control and Prevention (NIOSH/CDC), and the National Center for Toxicological Research of the Food and Drug Administration (NCTR/FDA). Established in 1978, the NTP is charged with coordinating toxicological testing activities, strengthening the science base in toxicology, developing and validating improved testing methods, and providing information about potentially toxic substances to health regulatory and research agencies, scientific and medical communities, and the public. The Technical Report series began in 1976 with carcinogenesis studies conducted by the National Cancer Institute. In 1981, this bioassay program was transferred to the NTP. The studies described in the Technical Report series are designed and conducted to characterize and evaluate the toxicologic potential, including carcinogenic activity, of selected substances in laboratory animals (usually two species, rats and mice). Substances selected for NTP toxicity and carcinogenicity studies are chosen primarily on the basis of human exposure, level of production, and chemical structure. -

Steroidogenic Pathway Poster-COMPLEX-24X36
Cholesterol The Steroidogenic Pathways Congenital adrenal hyperplasia (CAH), Hyperglycemia, azole antifungals, aging, smoking, E2, progesterone, azole smoking, dioxin toxicity, HIV, licorice, Phytoestrogens, flaxseed, licorice, Alcohol, IL-6, abdominal obesity, DHEA, antifungals, spironolactone etomidate, liarozole progestins, tamoxifen forskolin (-) (-) (-) (+) Pregnenolone CYPc17 17-OH-Pregnenolone 17,20 Lyase DHEAA17βHSD ndrostenediol (+) Hyperglycemia, hyperinsulinemia, PCOS (ovary), stress, alcohol, antiepileptics (+) CAH, chronic ETOH ingestion, PCB toxicity, progestins, D D D (-) (-) D PCB toxicity, DHEA, antiepileptics isoflavonoids, metformin, troglitazone, trilostane HS Pregnanediol Pregnanetriol β HS HS 3 HS β β β 3 3 3 (+) Hyperadrenalism, hyperthyroidism, hyperinsulinemia, PCOS, IL-4 (+) and IL-13, IGF-1, forskolin Smoking, dioxin, isoflavones, flaxseed, procyanidins, flavonoids, EGCG, epilobium, vitamin C, anastrazole, etc; CYPc17 17,20 Lyase 17βHSD Progesterone 17-OH-Progesterone Androstenedione Testosterone chrysin, stinging nettles, (-) and other flavonoids; β 5 ketoconazole, metformin α (+) (+) 1 5 1 Sodium depletion, high prolactin More cortisol: Less cortisol: Etiocholanolone Obesity (esp. visceral), A Good insulin sensitivity, ro CAH, late-onset adrenal hyperplasia, primary adrenal CYP2 metabolic A CYP2 hyperthyroidism, ro 5 m Excess adipose, alcohol, zinc (-) insufficiency, ankylosing spondylitis, increased salt (-) syndrome, hypothyroid, m α DHT at reduced inflammation, atas a (+) deficiency, stress, cravings, resveratrol, -

Steroid Metabolites Support Evidence of Autism As a Spectrum
behavioral sciences Article Steroid Metabolites Support Evidence of Autism as a Spectrum Benedikt Andreas Gasser 1,*, Johann Kurz 2, Bernhard Dick 1,3 and Markus Georg Mohaupt 1,4 1 Department of Clinical Research, University of Bern, 3010 Berne, Switzerland; [email protected] (B.D.); [email protected] (M.G.M.) 2 Intersci Research Association, Karl Morre Gasse 10, 8430 Leibnitz, Austria; [email protected] 3 Division of Nephrology/Hypertension, University of Bern, 3010 Berne, Switzerland 4 Teaching Hospital Internal Medicine, Lindenhofgruppe, 3006 Berne, Switzerland * Correspondence: [email protected] Received: 30 March 2019; Accepted: 6 May 2019; Published: 9 May 2019 Abstract: Objectives: It is common nowadays to refer to autism as a spectrum. Increased evidence of the involvement of steroid metabolites has been shown by the presence of stronger alterations in Kanner’s syndrome compared with Asperger syndrome. Methods: 24 h urine samples were collected from 20 boys with Asperger syndrome, 21 boys with Kanner’s syndrome, and identically sized control groups, each matched for age, weight, and height for comprehensive steroid hormone metabolite analysis via gas chromatography–mass spectrometry. Results: Higher levels of most steroid metabolites were detected in boys with Kanner’s syndrome and Asperger syndrome compared to their matched controls. These differences were more pronounced in affected individuals with Kanner’s syndrome versus Asperger syndrome. Furthermore, a specific and unique pattern of alteration of androsterone, etiocholanolone, progesterone, tetrahydrocortisone, and tetrahydrocortisol was identified in boys with Kanner’s syndrome and Asperger syndrome. Interestingly, in both matched samples, only androsterone, etiocholanolone, progesterone, tetrahydrocortisone, tetrahydrocortisol, and 5a-tetrahydrocortisol groups were positively correlated. -

Analysis of Anabolic Steroid Glucuronide and Sulfate Conjugates
Applicability of an innovative steroid-profiling method to determine synthetic growth promoter abuse in cattle Blokland, M.H.; Tricht, E.F. van; Ginkel L.A. van; Sterk, S.S. This is a "Post-Print" accepted manuscript, which has been published in “Catena” This version is distributed under a non-commencial no derivatives Creative Commons (CC-BY-NC-ND) user license, which permits use, distribution, and reproduction in any medium, provided the original work is properly cited and not used for commercial purposes. Further, the restriction applies that if you remix, transform, or build upon the material, you may not distribute the modified material. Please cite this publication as follows: Blokland, M.H.; Tricht, E.F. van; Ginkel L.A. van; Sterk, S.S. (2017) Applicability of an innovative steroid-profiling method to determine synthetic growth promoter abuse in cattle. The Journal of Steroid Biochemistry and Molecular Biology 174, p. 265-275 You can download the published version at: https://doi.org/10.1016/j.jsbmb.2017.10.007 Applicability of an innovative steroid-profiling method to determine synthetic growth promoter abuse in cattle 5 M.H. Blokland*, E.F. van Tricht, L.A van Ginkel, S.S. Sterk RIKILT Wageningen University & Research, P.O. Box 230, Wageningen, The Netherlands 10 *Corresponding author: M.H. Blokland, Tel.: +31 317 480417, E-mail: [email protected] 15 Keywords: synthetic steroids, growth promoters, cattle, UHPLC-MS/MS, steroid profiling, steroidogenesis Abstract 20 A robust LC-MS/MS method was developed to quantify a large number of phase I and phase II steroids in urine.