Increased and Mistimed Sex Hormone Production in Night Shift Workers
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
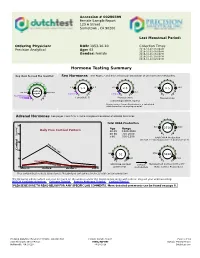
Hormone Testing Summary
Accession # 00280399 Female Sample Report 123 A Street Sometown , CA 90266 Last Menstrual Period: Ordering Physician: DOB: 1953-10-10 Collection Times: Precision Analytical Age: 63 2016-10-02 06:00AM 2016-10-02 08:00AM Gender: Female 2016-10-01 06:00PM 2016-10-01 10:00PM 2016-10-02 02:00AM Hormone Testing Summary Key (how to read the results): Sex Hormones See Pages 2 and 3 for a thorough breakdown of sex hormone metabolites opausa n l R e a m n e g 1.8 4.5 6.0 2.3 14.0 r 5.1 2.8 1.5 P e patient low limit high limit 20.0 result 0.2-0.7 0.3-2.0 Postmenopausal range Estradiol(E2) Progesterone Testosterone (Serum Equivalent, ng/mL) Progesterone Serum Equivalent is a calculated value based on urine pregnanediol. Adrenal Hormones See pages 4 and 5 for a more complete breakdown of adrenal hormones Total DHEA Production 300 500 3000 Age Range 2516 Daily Free Cortisol Pattern 20-39 1300-3000 240 40-60 750-2000 (ng/mg) >60 500-1200 Total DHEA Production (DHEAS + Etiocholanolone + Androsterone) 180 High Range Limit 120Cortisol 80 52 2750 5930 60 230 6500 Patient Values Low Range Limit 24hr Free Cortisol cortisol Metabolized Cortisol (THF+THE) 0 (A+B+C+D) metabolism (Total Cortisol Production) Waking (A) Morning (B) Afternoon (C) Night (D) Free cortisol best reflects tissue levels. Metabolized cortisol best reflects total cortisol production. The following videos (which can also be found on the website under the listed names along with others) may aid your understanding: DUTCH Complete Overview Estrogen Tutorial Female Androgen Tutorial Cortisol Tutorial PLEASE BE SURE TO READ BELOW FOR A NY SPECIFIC LA B COMMENTS. -

The Steroid Metabolome in Men with Mood and Anxiety Disorders
Physiol. Res. 64 (Suppl. 2): S275-S282, 2015 https://doi.org/10.33549/physiolres.933067 The Steroid Metabolome in Men With Mood and Anxiety Disorders M. DUŠKOVÁ1, M. HILL1, M. BIČÍKOVÁ1, M. ŠRÁMKOVÁ1, D. ŘÍPOVÁ2, P. MOHR2, L. STÁRKA1 1Institute of Endocrinology, Prague, Czech Republic, 2National Institute of Mental Health, Klecany, Czech Republic Received May 5, 2015 Accepted May 20, 2015 Summary many other hormones and various factors have been The mood and behavior of individuals result from an orchestra of identified as modulators of mood and behavior in such many factors. Among them steroids play an important role; disorders. Recent studies have described the role of brain- however, only several common hormones have been investigated derived neurotrophic factor (BDNF) (Pluchino et al. in this respect. It has been demonstrated that some steroid 2013, Numakawa et al. 2014), thyroid hormones (Duntas metabolites long considered merely the products of steroid and Mails 2013), inflammation (Halaris 2013), immunity hormone metabolism in fact possess considerable activity in the (Pitychoutis and Papadopoulou-Daifoti 2010), melatonin CNS. For this reason we studied the steroid metabolome (Boyce and Hopwood 2013), oxytocin and vasopressin including 50 analytes in 20 men with depression, 20 men with (Scantamburlo et al. 2009, Matsuzaki et al. 2012), the anxiety and 30 healthy controls. Significant differences were renin-angiotensin-aldosterone system (Franklin et al. found not only between controls and men with either depression 2012, Murck et al. 2012), the cannabinoid system or anxiety, but also between men with depression and anxiety. (Martykánová 2010, Gorzalka and Hill 2011, Smaga et Particularly striking were those steroids until now not generally al. -

Studies on Steroid Fever II. Pyrogenic and Anti-Pyrogenic Activity in Vitro of Some Endogenous Steroids of Man
Studies on steroid fever II. Pyrogenic and anti-pyrogenic activity in vitro of some endogenous steroids of man G. M. Dillard, Phyllis Bodel J Clin Invest. 1970;49(12):2418-2426. https://doi.org/10.1172/JCI106461. Research Article The pyrogenic properties of some C-19 and C-21 steroids were examined by in vitro incubation of human blood leukocytes with serum-buffer solutions of the steroids and injection of the 18-hr supernatants into rabbits. In previous studies this method demonstrated release of leukocyte endogenous pyrogen by etiocholanolone. With two exceptions, steroids known to cause fever in man, such as 11β-OH etiocholanolone and 3α-hydroxy-5β-pregnane-20-one were also pyrogenic in vitro. All steroids tested which are nonpyrogenic in man, such as androsterone, 3β-OH etiocholanolone, and 3α, 17α-dihydroxy-5β-pregnan-20-one were also nonpyrogenic in vitro. Solubility in aqueous solution did not correlate with pyrogenic capacity. Inhibition of pyrogen release from human leukocytes in vitro by hydrocortisone and estradiol was demonstrated. Hydrocortisone-treated leukocytes released less pyrogen than did normal leukocytes when stimulated either by etiocholanolone or by phagocytosis of heat-killed staphylococci. On the other hand, estradiol-treated blood leukocytes and mononuclear cells showed significant suppression of pyrogen release when phagocytosis, but not etiocholanolone, was used as the stimulus. When blood cells were incubated with progesterone, greater than normal amounts of pyrogen were released following phagocytosis, and the inhibiting effect of estradiol could be partially reversed. Neither estradiol nor hydrocortisone appeared to act on rabbit leukocytes. These studies indicate that a variety of naturally-occurring steroids may alter pyrogen release from leukocytes. -

AOD Biennial Review
Introduction The Drug-Free Schools and Communities Act and Part 86 of the Education Department General Administrative Regulations (EDGAR) require each institution of higher education participating in Title IV student financial assistance to certify that it has developed and implemented a program to prevent the unlawful possession, use, and distribution of drugs and alcohol on campus and at institution-recognized events and activities. Annually, the institution must distribute to current students and employees written information about its drug and alcohol abuse prevention program, including information about related sanctions. The distribution plan must include provisions for sharing this information with new students and employees who join the institution at later points in the year as well. In addition, on a biennial basis, the institution must conduct a review to determine the effectiveness of its programs and prepare a report of the review’s findings. The report must be retained and made available to the US Department of Education upon request. Notre Dame of Maryland University (“NDMU” or “the University”) acknowledges these responsibilities, including the timely review of and reporting on the effectiveness of its prevention programs. NDMU is a private, Catholic university established in 1895 with the mission to educate leaders to transform the world. NDMU is a comprehensive university that is home to Maryland's only women's college and offers a wide variety of full and part-time undergraduate, graduate, doctoral, and certificate programs to women and men. The University enrolls approximately 2300 students and has Schools of Arts, Sciences, and Business; Education; Nursing; and Pharmacy. Additionally, the University offers programs for adult students fully online and at several locations throughout the State. -

Quantification of Hair Corticosterone, DHEA and Testosterone As
animals Article Quantification of Hair Corticosterone, DHEA and Testosterone as a Potential Tool for Welfare Assessment in Male Laboratory Mice Alberto Elmi 1 , Viola Galligioni 2, Nadia Govoni 1 , Martina Bertocchi 1 , Camilla Aniballi 1 , Maria Laura Bacci 1 , José M. Sánchez-Morgado 2 and Domenico Ventrella 1,* 1 Department of Veterinary Medical Sciences, University of Bologna, 40064 Ozzano dell’Emilia, BO, Italy; [email protected] (A.E.); [email protected] (N.G.); [email protected] (M.B.); [email protected] (C.A.); [email protected] (M.L.B.) 2 Comparative Medicine Unit, Trinity College Dublin, D02 Dublin, Ireland; [email protected] (V.G.); [email protected] (J.M.S.-M.) * Correspondence: [email protected]; Tel.: +39-051-2097-926 Received: 11 November 2020; Accepted: 14 December 2020; Published: 16 December 2020 Simple Summary: Mice is the most used species in the biomedical research laboratory setting. Scientists are constantly striving to find new tools to assess their welfare, in order to ameliorate husbandry conditions, leading to a better life and scientific data. Steroid hormones can provide information regarding different behavioral tracts of laboratory animals but their quantification often require stressful sampling procedures. Hair represents a good, less invasive, alternative in such scenario and is also indicative of longer timespan due to hormones’ accumulation. The aim of the work was to quantify steroid hormones in the hair of male laboratory mice and to look for differences imputable to age and housing conditions (pairs VS groups). Age influenced all analysed hormones by increasing testosterone and dehydroepiandrosterone (DHEA) levels and decreasing corticosterone. -

Alteration of the Steroidogenesis in Boys with Autism Spectrum Disorders
Janšáková et al. Translational Psychiatry (2020) 10:340 https://doi.org/10.1038/s41398-020-01017-8 Translational Psychiatry ARTICLE Open Access Alteration of the steroidogenesis in boys with autism spectrum disorders Katarína Janšáková 1, Martin Hill 2,DianaČelárová1,HanaCelušáková1,GabrielaRepiská1,MarieBičíková2, Ludmila Máčová2 and Daniela Ostatníková1 Abstract The etiology of autism spectrum disorders (ASD) remains unknown, but associations between prenatal hormonal changes and ASD risk were found. The consequences of these changes on the steroidogenesis during a postnatal development are not yet well known. The aim of this study was to analyze the steroid metabolic pathway in prepubertal ASD and neurotypical boys. Plasma samples were collected from 62 prepubertal ASD boys and 24 age and sex-matched controls (CTRL). Eighty-two biomarkers of steroidogenesis were detected using gas-chromatography tandem-mass spectrometry. We observed changes across the whole alternative backdoor pathway of androgens synthesis toward lower level in ASD group. Our data indicate suppressed production of pregnenolone sulfate at augmented activities of CYP17A1 and SULT2A1 and reduced HSD3B2 activity in ASD group which is partly consistent with the results reported in older children, in whom the adrenal zona reticularis significantly influences the steroid levels. Furthermore, we detected the suppressed activity of CYP7B1 enzyme readily metabolizing the precursors of sex hormones on one hand but increased anti-glucocorticoid effect of 7α-hydroxy-DHEA via competition with cortisone for HSD11B1 on the other. The multivariate model found significant correlations between behavioral indices and circulating steroids. From dependent variables, the best correlation was found for the social interaction (28.5%). Observed changes give a space for their utilization as biomarkers while reveal the etiopathogenesis of ASD. -

Effect of Maternal Intrahepatic Cholestasis on Fetal Steroid Metabolism
Effect of Maternal Intrahepatic Cholestasis on Fetal Steroid Metabolism Timo J. Laatikainen, … , Jari I. Peltonen, Pekka L. Nylander J Clin Invest. 1974;53(6):1709-1715. https://doi.org/10.1172/JCI107722. Research Article Estriol, estriol sulfate, progesterone, and 17 neutral steroid sulfates, including estriol precursors and progesterone metabolites, were determined in 27 cord plasma samples collected after pregnancies complicated by intrahepatic cholestasis of the mother. The levels of these steroids were compared with those in the cord plasma of 42 healthy controls. In the cord plasma, the steroid profile after pregnancies complicated by maternal intrahepatic cholestasis differed greatly from that seen after uncomplicated pregnancy. Two main differences were found. In the disulfate fraction, the concentrations of two pregnanediol isomers, 5α-pregnane-3α,20α-diol and 5β-pregnane-3α,20α-diol, were high after cholestasis. Other investigators have shown that, as a result of cholestasis, these pregnanediol sulfates circulate in greatly elevated amounts in the maternal plasma. Our results indicate that in cholestasis these steroids cross the placenta into the fetal compartment, where they circulate in elevated amounts as disulfates. Secondly, the concentrations of several steroid sulfates known to be synthesized by the fetus were significantly lower in the cholestasis group than in the healthy controls. This was especially true of 16α-hydroxydehydroepiandrosterone sulfate and 16α- hydroxypregnenolone sulfate. These results suggest that, in pregnancies complicated by maternal intrahepatic cholestasis, impairment of fetal steroid synthesis, and especially of 16α-hydroxylation, occurs in the fetal compartment. Thus, the changes in maternal steroid metabolism caused by cholestasis are reflected in the steroid profile of the fetoplacental circulation. -

Metabolism of Testoterone and Related Steroids in Metastatic Interstitial Cell Carcinoma of the Testis
Metabolism of testoterone and related steroids in metastatic interstitial cell carcinoma of the testis. M B Lipsett, … , C W Bardin, L M Fishman J Clin Invest. 1966;45(11):1700-1709. https://doi.org/10.1172/JCI105476. Research Article Find the latest version: https://jci.me/105476/pdf Journal of Clinical Investigation Vol. 45, No. 11, 1966 Metabolism of Testosterone and Related Steroids in Metastatic Interstitial Cell Carcinoma of the Testis * M. B. LIPSETT,t G. A. SARFATY, H. WILSON, C. WAYNE BARDIN, AND L. M. FISHMAN (From the Endocrinology Branch, National Cancer Institute, Bethesda, Md.) Interstitial cell carcinoma of the testis is a singu- production rate has been shown to be a conse- larly rare steroid-producing cancer. Of the seven quence of metabolism of dehydroepiandrosterone reported cases (1-7), urinary 17-ketosteroid (17- sulfate. KS) excretion was high in the four cases in which it was measured. Abelson, Bulaschenko, Trom- Methods mer, and Valdes-Dapena (7) fractionated the uri- Routine methods were used to analyze the following: nary 17-ketosteriods and corticoids in one recently urinary 17-KS (8), urinary 17-hydroxycorticoids (9), reported case. There is, however, no comprehen- plasma Silber-Porter chromogens (10), and plasma tes- tosterone (11). sive study of either the production of androgens Gas-liquid chromatography. We carried out gas-liquid or related steroids by this tumor. We have had chromatography (GLC) in a Glowell Chromolab gas the opportunity to study a patient with metastatic chromatograph utilizing a 'Sr ionization detector oper- interstitial cell carcinoma, and we have examined ating at 1,050 v. -

PDG) Enzyme Immunoassay Kit
DetectX® Pregnanediol-3-Glucuronide (PDG) Enzyme Immunoassay Kit 1 Plate Kit Catalog Number K037-H1 5 Plate Kit Catalog Number K037-H5 Species Independent Sample Types Validated: Dried Fecal Extracts, Urine, Extracted Serum/Plasma, and Tissue Culture Media Please read this insert completely prior to using the product. For research use only. Not for use in diagnostic procedures. www.ArborAssays.com K037-H WEB 210301 TABLE OF CONTENTS Background 3 Assay Principle 4 Related Products 4 Supplied Components 5 Storage Instructions 5 Other Materials Required 6 Precautions 6 Sample Types 7 Sample Preparation 7 Reagent Preparation 8 Assay Protocol 9 Calculation of Results 10 Typical Data 10-11 Validation Data Sensitivity, Linearity, etc. 11-13 Samples Values and Cross Reactivity 14 Warranty & Contact Information 15 Plate Layout Sheet 16 ® 2 EXPECT ASSAY ARTISTRY™ K037-H WEB 210301 BACKGROUND Pregnanediol Glucuronide, C27H44O8, also known as PDG (5β-Pregnan-3a,20a-diol 3-glucosiduronate) is the major metabolite of progesterone1-4. Progesterone is the hormone involved in the female menstrual cycle, gestation and embryogenesis of humans and other species. Progesterone belongs to a class of hormones called progestogens, and is the major naturally occurring human progestogen5,6. Progesterone is an essential regulator of human female reproductive function in the uterus, ovary, mammary gland and brain, and plays an important role in non-reproductive tissues such as the cardiovascular system, bone and the central nervous system. Progesterone action is conveyed by two isoforms of the nuclear progesterone receptor (PR), PRA and PRB. PRA and B are expressed in a variety of normal breast tissue from humans, rats and mice and is also expressed in breast cancer cells7,8. -

Steroid Metabolites Support Evidence of Autism As a Spectrum
behavioral sciences Article Steroid Metabolites Support Evidence of Autism as a Spectrum Benedikt Andreas Gasser 1,*, Johann Kurz 2, Bernhard Dick 1,3 and Markus Georg Mohaupt 1,4 1 Department of Clinical Research, University of Bern, 3010 Berne, Switzerland; [email protected] (B.D.); [email protected] (M.G.M.) 2 Intersci Research Association, Karl Morre Gasse 10, 8430 Leibnitz, Austria; [email protected] 3 Division of Nephrology/Hypertension, University of Bern, 3010 Berne, Switzerland 4 Teaching Hospital Internal Medicine, Lindenhofgruppe, 3006 Berne, Switzerland * Correspondence: [email protected] Received: 30 March 2019; Accepted: 6 May 2019; Published: 9 May 2019 Abstract: Objectives: It is common nowadays to refer to autism as a spectrum. Increased evidence of the involvement of steroid metabolites has been shown by the presence of stronger alterations in Kanner’s syndrome compared with Asperger syndrome. Methods: 24 h urine samples were collected from 20 boys with Asperger syndrome, 21 boys with Kanner’s syndrome, and identically sized control groups, each matched for age, weight, and height for comprehensive steroid hormone metabolite analysis via gas chromatography–mass spectrometry. Results: Higher levels of most steroid metabolites were detected in boys with Kanner’s syndrome and Asperger syndrome compared to their matched controls. These differences were more pronounced in affected individuals with Kanner’s syndrome versus Asperger syndrome. Furthermore, a specific and unique pattern of alteration of androsterone, etiocholanolone, progesterone, tetrahydrocortisone, and tetrahydrocortisol was identified in boys with Kanner’s syndrome and Asperger syndrome. Interestingly, in both matched samples, only androsterone, etiocholanolone, progesterone, tetrahydrocortisone, tetrahydrocortisol, and 5a-tetrahydrocortisol groups were positively correlated. -

NON-CLINICAL REVIEW(S) DEPARTMENT of HEALTH & HUMAN SERVICES Food and Drug Administration
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 206089Orig1s000 NON-CLINICAL REVIEW(S) DEPARTMENT OF HEALTH & HUMAN SERVICES Food and Drug Administration PHARMACOLOGY/TOXICOLOGY MEMORANDUM CDER Stamp Date: September 27, 2018 NDA: 206089 Sponsor: Clarus Therapeutics, Inc. Drug: Testosterone undecanoate (JATENZO) Replacement therapy in adult males for conditions associated Indication: with a deficiency or absence of endogenous testosterone due to primary or hypogonadotropic hypogonadism Subject: Final Labeling Review Reviewer: Yangmee Shin, PhD Background: Clarus Therapeutics resubmitted NDA 206089 under a 505(b)(2) regulatory pathway following a 2nd Complete Response (CR) letter issued on March 22, 2018. NDA 206089 was first submitted on January 3, 2014 as a 505(b)(2) application. To support the nonclinical requirements of NDA via a 505(b)(2) pathway, the sponsor submitted published literature along with the findings of a 3-month oral toxicology study of Clarus’ oral testosterone undecanoate (TU) formulation in dogs. The sponsor also provided literature references to address ADME of TU by the oral route. The 3-month oral toxicology study in male dogs and relevant published literature were provided to support the use of borage oil as a novel excipient. The sponsor also supplied published literature regarding the fertility, pregnancy, and carcinogenicity of testosterone (T). Pharmacology and Toxicology recommended approval of the initial submission of NDA 206089 during the first cycle review. In the 1st resubmission to NDA 206089 on June 22, 2017, Clarus refiled the NDA as a 505(b)(1) application and provided nonclinical studies of oral TU including a 9-month oral toxicology study in male dogs, a battery of genotoxicity tests, a 6-month carcinogenicity study in Tg·rasH2 male mice, and a fertility study in male rats, upon agreement with the Division on November 19, 2015. -

Sample Report TST
PATIENT: Sample Report TEST REF: TST-##-##### TEST NUMBER: ########### RECEIVED: d-mm-yyyy PRACTITIONER: Nordic Laboratories PATIENT NUMBER: ############ TESTED: d-mm-yyyy GENDER: Female COLLECTED: d-mm-yyyy h:m ADDRESS: AGE: 46 DATE OF BIRTH: d-mm-yyyy TEST NAME: Urinary Hormone Metabolites Estrogen Elite Test Name Result Range Urinary Estrogens Estradiol 3.71 H 0.78-1.79 µg/g Cr Premeno-luteal or ERT Estrone 10.95 H 2.27-5.22 µg/g Cr Premeno-luteal or ERT Estriol 3.19 H 0.78-1.98 µg/g Cr Premeno-luteal or ERT E3/(E1+E2) 0 22 L >0.3 (> median value) 2-OH Estradiol 0.77 H 0.17-0.70 µg/g Cr Premeno-luteal or ERT 2-OH Estrone 2.49 0.70-2.54 µg/g Cr Premeno-luteal or ERT 4-OH Estradiol 0.45 H 0.10-0.18 µg/g Cr Premeno-luteal or ERT 4-OH Estrone 0.64 H 0.17-0.47 µg/g Cr Premeno-luteal or ERT 16α-OH Estrone 2 25 H 0.35-1.07 µg/g Cr Premeno-luteal or ERT 2-OH (E1 + E2)/16-α-OH E1 1.45 1.29-5.49 Premeno-luteal or ERT 2-MeO Estradiol 0.11 H 0.03-0.08 µg/g Cr Premeno-luteal or ERT 2-MeO Estrone 0.83 H 0.26-0.68 µg/g Cr Premeno-luteal or ERT 2-MeO E1/2-OH E1 0.33 0.21-0.38 Premeno-luteal or ERT 4-MeO Estradiol 0.02 <0.04 µg/g Cr 4-MeO Estrone 0.05 H <0.04 µg/g Cr 4-MeO E1/4-OH E1 0.08 0.05-0.13 Premeno-luteal or ERT 4-MeO E2/4-OH E2 0.04 L 0.10-0.29 Premeno-luteal or ERT Bisphenol A 1.61 1.11-3.74 µg/g Cr Premeno-luteal Urinary Progestogens Pregnanediol 201 L 465-1609 µg/g Cr Premeno-luteal or PgRT Allopregnanolone 2.05 L 2.23-14.87 µg/g Cr Premeno-luteal or PgRT Pgdiol/E2 54.18 L 1000-1500 (Optimal Luteal Only) Urinary Androgens DHEA 50.43 15.82-129.17 µg/g Cr Premeno-luteal or DHEAT Androstenedione 5.18 3.93-13.53 µg/g Cr Premeno-luteal or ART Testosterone 0.44 L 1.22-3.97 µg/g Cr Premeno-luteal or ART Epi-Testosterone 2.57 2.01-4.66 µg/g Cr Premeno-luteal T/Epi-T 0.17 L 0.5-3.0 5α-DHT 0.12 L 0.28-1.52 µg/g Cr Premeno-luteal or ART Urinary Creatinine Creatinine (pooled) 1 26 0.3-2.0 mg/mL Nordic Laboratories Aps UK Office: Page 1 of 7 Nygade 6, 3.sal • 1164 Copenhagen K • Denmark 11 Old Factory Buildings • Stonegate • E.