Disappearance and Conjugation of Free Etiocholanolone Peter Berkeley Gregory Yale University
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
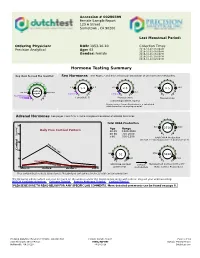
Hormone Testing Summary
Accession # 00280399 Female Sample Report 123 A Street Sometown , CA 90266 Last Menstrual Period: Ordering Physician: DOB: 1953-10-10 Collection Times: Precision Analytical Age: 63 2016-10-02 06:00AM 2016-10-02 08:00AM Gender: Female 2016-10-01 06:00PM 2016-10-01 10:00PM 2016-10-02 02:00AM Hormone Testing Summary Key (how to read the results): Sex Hormones See Pages 2 and 3 for a thorough breakdown of sex hormone metabolites opausa n l R e a m n e g 1.8 4.5 6.0 2.3 14.0 r 5.1 2.8 1.5 P e patient low limit high limit 20.0 result 0.2-0.7 0.3-2.0 Postmenopausal range Estradiol(E2) Progesterone Testosterone (Serum Equivalent, ng/mL) Progesterone Serum Equivalent is a calculated value based on urine pregnanediol. Adrenal Hormones See pages 4 and 5 for a more complete breakdown of adrenal hormones Total DHEA Production 300 500 3000 Age Range 2516 Daily Free Cortisol Pattern 20-39 1300-3000 240 40-60 750-2000 (ng/mg) >60 500-1200 Total DHEA Production (DHEAS + Etiocholanolone + Androsterone) 180 High Range Limit 120Cortisol 80 52 2750 5930 60 230 6500 Patient Values Low Range Limit 24hr Free Cortisol cortisol Metabolized Cortisol (THF+THE) 0 (A+B+C+D) metabolism (Total Cortisol Production) Waking (A) Morning (B) Afternoon (C) Night (D) Free cortisol best reflects tissue levels. Metabolized cortisol best reflects total cortisol production. The following videos (which can also be found on the website under the listed names along with others) may aid your understanding: DUTCH Complete Overview Estrogen Tutorial Female Androgen Tutorial Cortisol Tutorial PLEASE BE SURE TO READ BELOW FOR A NY SPECIFIC LA B COMMENTS. -

Excretion Patterns of Fecal Progestagens, Androgen and Estrogens During Pregnancy, Parturition and Postpartum in Okapi (Okapia Johnstoni)
Journal of Reproduction and Development, Vol. 53, No. 1, 2007 —Research Note— Excretion Patterns of Fecal Progestagens, Androgen and Estrogens During Pregnancy, Parturition and Postpartum in Okapi (Okapia johnstoni) Satoshi KUSUDA1), Koki MORIKAKU2), Ken-ichi KAWADA3), Kenji ISHIWADA4)# and Osamu DOI3) 1)Laboratory of Animal Reproduction, United Graduate School of Agricultural Science, Gifu University, Gifu 501-1193, 2)Preservation and Research Center, City of Yokohama, Kanagawa 241-0804, 3)Laboratory of Animal Reproduction, Faculty of Applied Biological Sciences, Gifu University, Gifu 501-1193 and 4)Yokohama Zoological Gardens Zoorasia, Kanagawa 241- 0001, Japan #Present: Kanazawa Zoological Gardens of Yokohama, Kanagawa 236-0042, Japan Abstract. The aim of the present study was to establish a simple method to monitor ovarian activity and non-invasively diagnose pregnancy in okapi (Okapia johnstoni). The feces of a female okapi were collected daily or every 3 days for 28 months. Steroids in lyophilized feces were extracted with 80% methanol, and the fecal levels of immunoreactive progestagens (progesterone and pregnanediol- glucuronide), androgen (testosterone), and estrogens (estradiol-17β and estrone) were determined by enzyme immunoassays with commercially available antisera. Using the progesterone profiles, the durations of the luteal phase, follicular phase, and estrous cycle were determined to be 11.1 ± 0.4, 5.3 ± 0.6, and 16.5 ± 0.7 days (n=22), respectively. Fecal levels of immunoreactive progesterone, pregnanediol glucuronide, and testosterone gradually increased from early pregnancy and peaked several months before parturition. More pregnanediol glucuronide was excreted in feces than progesterone during late pregnancy, but not during the estrous cycle. Although the fecal concentrations of immunoreactive estradiol-17β and estrone change a little throughout pregnancy and non-pregnancy, they rose sharply and temporarily on the day following parturition. -

01 Front.Pdf
Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and private study only. The thesis may not be reproduced elsewhere without the permission of the Author. STUDIES TOWARDS THE DEVELOPMENT OF A MULTI PURPOSE HOME SELF-TEST KIT FOR THE DETECTION OF URINARY TETRAHYDROCORTISONE AND TESTOSTERONE METABOLITES A thesis submitted in partial fulfilment of the requirements for the degree of Master of Science in Chemistry at Massey University Claire Margaret Nielsen 2003 ii Abstract The development of homogeneous enzyme immunoassays (HEIA) for testosterone glucuronide (TG) and tetrahydrocortisone glucuronide (THEG) in urine are described. The proposed test system is based on the Ovarian Monitor homogeneous immunoassay system, established by J.B Brown and L.F. Blackwell et al. 1 as a simple, laboratory accurate, monitoring device for the measurement of estrone glucuronide (E1G) and pregnanediol glucuronide (PdG) as markers of the fertile phase during a womans menstrual cycle. This information can be used readily by women to identify their cyclical periods of fertility and infertility. The major testosterone metabolite in the urine of males, testosterone p-glucuronide, was synthesised by firstly preparing the glycosyl donor a-bromosugar and conjugating this with testosterone under standard Koenigs-Knorr conditions. 1H nmr studies confirmed that the synthetic steroid glucuronide had the same stereochemistry as the naturally occurring urinary testosterone glucuronide. Testosterone glucuronide and tetrahydrocortisone glucuronide conjugates of hen egg white lysozyme were prepared using the active ester coupling method in good yield. Unreacted lysozyme was successfully removed from the reaction mixture by a combination of cation exchange chromatography in 7 M urea and hydrophobic-interaction chromatography. -

The Steroid Metabolome in Men with Mood and Anxiety Disorders
Physiol. Res. 64 (Suppl. 2): S275-S282, 2015 https://doi.org/10.33549/physiolres.933067 The Steroid Metabolome in Men With Mood and Anxiety Disorders M. DUŠKOVÁ1, M. HILL1, M. BIČÍKOVÁ1, M. ŠRÁMKOVÁ1, D. ŘÍPOVÁ2, P. MOHR2, L. STÁRKA1 1Institute of Endocrinology, Prague, Czech Republic, 2National Institute of Mental Health, Klecany, Czech Republic Received May 5, 2015 Accepted May 20, 2015 Summary many other hormones and various factors have been The mood and behavior of individuals result from an orchestra of identified as modulators of mood and behavior in such many factors. Among them steroids play an important role; disorders. Recent studies have described the role of brain- however, only several common hormones have been investigated derived neurotrophic factor (BDNF) (Pluchino et al. in this respect. It has been demonstrated that some steroid 2013, Numakawa et al. 2014), thyroid hormones (Duntas metabolites long considered merely the products of steroid and Mails 2013), inflammation (Halaris 2013), immunity hormone metabolism in fact possess considerable activity in the (Pitychoutis and Papadopoulou-Daifoti 2010), melatonin CNS. For this reason we studied the steroid metabolome (Boyce and Hopwood 2013), oxytocin and vasopressin including 50 analytes in 20 men with depression, 20 men with (Scantamburlo et al. 2009, Matsuzaki et al. 2012), the anxiety and 30 healthy controls. Significant differences were renin-angiotensin-aldosterone system (Franklin et al. found not only between controls and men with either depression 2012, Murck et al. 2012), the cannabinoid system or anxiety, but also between men with depression and anxiety. (Martykánová 2010, Gorzalka and Hill 2011, Smaga et Particularly striking were those steroids until now not generally al. -

Increased and Mistimed Sex Hormone Production in Night Shift Workers
Published OnlineFirst March 3, 2015; DOI: 10.1158/1055-9965.EPI-14-1271 Research Article Cancer Epidemiology, Biomarkers Increased and Mistimed Sex Hormone Production & Prevention in Night Shift Workers Kyriaki Papantoniou1,2,3,4, Oscar J. Pozo2, Ana Espinosa1,2,3,4, Josep Marcos2,3, Gemma Castano-Vinyals~ 1,2,3,4, Xavier Basagana~ 1,2,3,4, Elena Juanola Pages 5, Joan Mirabent6,7, Jordi Martín8, Patricia Such Faro9, Amparo Gasco Aparici10, Benita Middleton11, Debra J. Skene11, and Manolis Kogevinas1,2,3,4,12 Abstract Background: Night shift work has been associated with Results: Night workers had higher levels of total progestagens an increased risk for breast and prostate cancer. The effect [geometric mean ratio (GMR) 1.65; 95% confidence intervals of circadian disruption on sex steroid production is a pos- (CI), 1.17–2.32] and androgens (GMR: 1.44; 95% CI, 1.03–2.00), sible underlying mechanism, underinvestigated in hum- compared with day workers, after adjusting for potential con- ans. We have assessed daily rhythms of sex hormones founders. The increased sex hormone levels among night and melatonin in night and day shift workers of both shift workers were not related to the observed suppression of sexes. 6-sulfatoxymelatonin. Peak time of androgens was significantly Methods: We recruited 75 night and 42 day workers, ages later among night workers, compared with day workers (testos- 22 to 64 years, in different working settings. Participants terone: 12:14 hours; 10:06-14:48 vs. 08:35 hours; 06:52-10:46). collected urine samples from all voids over 24 hours on a Conclusions: We found increased levels of progestagens and working day. -

Studies on Steroid Fever II. Pyrogenic and Anti-Pyrogenic Activity in Vitro of Some Endogenous Steroids of Man
Studies on steroid fever II. Pyrogenic and anti-pyrogenic activity in vitro of some endogenous steroids of man G. M. Dillard, Phyllis Bodel J Clin Invest. 1970;49(12):2418-2426. https://doi.org/10.1172/JCI106461. Research Article The pyrogenic properties of some C-19 and C-21 steroids were examined by in vitro incubation of human blood leukocytes with serum-buffer solutions of the steroids and injection of the 18-hr supernatants into rabbits. In previous studies this method demonstrated release of leukocyte endogenous pyrogen by etiocholanolone. With two exceptions, steroids known to cause fever in man, such as 11β-OH etiocholanolone and 3α-hydroxy-5β-pregnane-20-one were also pyrogenic in vitro. All steroids tested which are nonpyrogenic in man, such as androsterone, 3β-OH etiocholanolone, and 3α, 17α-dihydroxy-5β-pregnan-20-one were also nonpyrogenic in vitro. Solubility in aqueous solution did not correlate with pyrogenic capacity. Inhibition of pyrogen release from human leukocytes in vitro by hydrocortisone and estradiol was demonstrated. Hydrocortisone-treated leukocytes released less pyrogen than did normal leukocytes when stimulated either by etiocholanolone or by phagocytosis of heat-killed staphylococci. On the other hand, estradiol-treated blood leukocytes and mononuclear cells showed significant suppression of pyrogen release when phagocytosis, but not etiocholanolone, was used as the stimulus. When blood cells were incubated with progesterone, greater than normal amounts of pyrogen were released following phagocytosis, and the inhibiting effect of estradiol could be partially reversed. Neither estradiol nor hydrocortisone appeared to act on rabbit leukocytes. These studies indicate that a variety of naturally-occurring steroids may alter pyrogen release from leukocytes. -

Center for Studies in Demography and Ecology
Page 1 K.A. O’Connor Center for Studies in Demography and Ecology Urinary enzyme-immunoassays for population research on reproduction: Estrone conjugates and pregnanediol-3-gluceronide by Kathleen O’Connor University of Washington UNIVERSITY OF WASHINGTON CSDE Working Paper No. 01-11 Page 2 K.A. O’Connor Title: Urinary enzyme-immunoassays for population research on reproduction: Estrone conjugates and pregnanediol-3-glucuronide. Running Title: Urinary EIA’s for population research: E1C and PDG Authors and Institutions: Kathleen A. O’Connor1 Eleanor Brindle1 Darryl J. Holman1 Nancy A. Klein 2 Michael R. Soules 2 Kenneth L. Campbell 3 Fortüne Kohen 4 Coralie J. Munro 5 William L. Lasley 6 James W. Wood 7 1 Department of Anthropology and Center for Studies in Demography and Ecology, University of Washington, Seattle WA 98195 2 Department of Obstetrics and Gynecology, University of Washington, Seattle WA 98195 3 Department of Biology, University of Massachusetts, Boston MA 02125 4 Department of Biological Regulation, Weizmann Institute of Science, Rehovet 76100 Israel 5 Department of Population Health and Reproduction, University of California, Davis, CA 95616 6 Department of Obstetrics and Gynecology, University of California, Davis, CA 95616 7 Department of Anthropology and Population Research Institute, Pennsylvania State University, University Park, PA 16802 Total Number of Pages:22 Number of Figures: 9 Number of Tables: 5 Keywords: E1G, E1C, EIA, PDG, urinary reproductive steroids, Quidel 330, validations, Bangladesh, 3F11 clone, 155B3 clone, assay validation, urine specimen stability, specific gravity Corresponding Author: Kathleen A. O’Connor Department of Anthropology Box 353100 University of Washington Seattle, Washington 98195 phone: (206) 543-9605 fax: (206) 543-3285 email: [email protected] Date: 10/19/2002 Page 3 K.A. -

Neurosteroids in Depression: a Review 39
PDF hosted at the Radboud Repository of the Radboud University Nijmegen The following full text is a publisher's version. For additional information about this publication click this link. http://hdl.handle.net/2066/71267 Please be advised that this information was generated on 2021-09-26 and may be subject to change. Frank van Broekhoven Effects of progesterone and allopregnanolone on stress, attention, cognition and mood | Frank van Broekhoven ISBN 978-90-9023655-1 Copyright ©2008 Frank van Broekhoven. The copyright of articles that have already been published has been transferred to the respective journals. No part of this book may be reproduced, in any form, without prior written permission from the author. Niets uit deze uitgave mag worden verveelvoudigd en/of openbaar gemaakt in welke vorm dan ook, zonder voorafgaande schriftelijke toestemming van de auteur. Coverdesign and layout by: Communicatie Kant, Dinxperlo, The Netherlands Printed by: Up2data, Bocholt, Germany The financial support for the printing of this thesis by Eli Lilly Nederland BV, Janssen-Cilag BV, the Department of Psychiatry from the Radboud University Nijmegen Medical Centre, and Karakter, Child and Adolescent Psy- chiatry University Centre, Nijmegen, is gratefully acknowledged. Effects of progesterone and allopregnanolone on stress, attention, cognition and mood Een wetenschappelijke proeve op het gebied van de Medische Wetenschappen Proefschrift ter verkrijging van de graad van doctor aan de Radboud Universiteit Nijmegen op gezag van de rector magnificus prof. mr. S.C.J.J. Kortmann, volgens besluit van het College van Decanen in het openbaar te verdedigen op maandag 24 november 2008 om 15.30 uur precies door Frank van Broekhoven geboren op 8 december 1969 te Groenlo Promotores: prof. -

AOD Biennial Review
Introduction The Drug-Free Schools and Communities Act and Part 86 of the Education Department General Administrative Regulations (EDGAR) require each institution of higher education participating in Title IV student financial assistance to certify that it has developed and implemented a program to prevent the unlawful possession, use, and distribution of drugs and alcohol on campus and at institution-recognized events and activities. Annually, the institution must distribute to current students and employees written information about its drug and alcohol abuse prevention program, including information about related sanctions. The distribution plan must include provisions for sharing this information with new students and employees who join the institution at later points in the year as well. In addition, on a biennial basis, the institution must conduct a review to determine the effectiveness of its programs and prepare a report of the review’s findings. The report must be retained and made available to the US Department of Education upon request. Notre Dame of Maryland University (“NDMU” or “the University”) acknowledges these responsibilities, including the timely review of and reporting on the effectiveness of its prevention programs. NDMU is a private, Catholic university established in 1895 with the mission to educate leaders to transform the world. NDMU is a comprehensive university that is home to Maryland's only women's college and offers a wide variety of full and part-time undergraduate, graduate, doctoral, and certificate programs to women and men. The University enrolls approximately 2300 students and has Schools of Arts, Sciences, and Business; Education; Nursing; and Pharmacy. Additionally, the University offers programs for adult students fully online and at several locations throughout the State. -

Alteration of the Steroidogenesis in Boys with Autism Spectrum Disorders
Janšáková et al. Translational Psychiatry (2020) 10:340 https://doi.org/10.1038/s41398-020-01017-8 Translational Psychiatry ARTICLE Open Access Alteration of the steroidogenesis in boys with autism spectrum disorders Katarína Janšáková 1, Martin Hill 2,DianaČelárová1,HanaCelušáková1,GabrielaRepiská1,MarieBičíková2, Ludmila Máčová2 and Daniela Ostatníková1 Abstract The etiology of autism spectrum disorders (ASD) remains unknown, but associations between prenatal hormonal changes and ASD risk were found. The consequences of these changes on the steroidogenesis during a postnatal development are not yet well known. The aim of this study was to analyze the steroid metabolic pathway in prepubertal ASD and neurotypical boys. Plasma samples were collected from 62 prepubertal ASD boys and 24 age and sex-matched controls (CTRL). Eighty-two biomarkers of steroidogenesis were detected using gas-chromatography tandem-mass spectrometry. We observed changes across the whole alternative backdoor pathway of androgens synthesis toward lower level in ASD group. Our data indicate suppressed production of pregnenolone sulfate at augmented activities of CYP17A1 and SULT2A1 and reduced HSD3B2 activity in ASD group which is partly consistent with the results reported in older children, in whom the adrenal zona reticularis significantly influences the steroid levels. Furthermore, we detected the suppressed activity of CYP7B1 enzyme readily metabolizing the precursors of sex hormones on one hand but increased anti-glucocorticoid effect of 7α-hydroxy-DHEA via competition with cortisone for HSD11B1 on the other. The multivariate model found significant correlations between behavioral indices and circulating steroids. From dependent variables, the best correlation was found for the social interaction (28.5%). Observed changes give a space for their utilization as biomarkers while reveal the etiopathogenesis of ASD. -

PDG) Enzyme Immunoassay Kit
DetectX® Pregnanediol-3-Glucuronide (PDG) Enzyme Immunoassay Kit 1 Plate Kit Catalog Number K037-H1 5 Plate Kit Catalog Number K037-H5 Species Independent Sample Types Validated: Dried Fecal Extracts, Urine, Extracted Serum/Plasma, and Tissue Culture Media Please read this insert completely prior to using the product. For research use only. Not for use in diagnostic procedures. www.ArborAssays.com K037-H WEB 210301 TABLE OF CONTENTS Background 3 Assay Principle 4 Related Products 4 Supplied Components 5 Storage Instructions 5 Other Materials Required 6 Precautions 6 Sample Types 7 Sample Preparation 7 Reagent Preparation 8 Assay Protocol 9 Calculation of Results 10 Typical Data 10-11 Validation Data Sensitivity, Linearity, etc. 11-13 Samples Values and Cross Reactivity 14 Warranty & Contact Information 15 Plate Layout Sheet 16 ® 2 EXPECT ASSAY ARTISTRY™ K037-H WEB 210301 BACKGROUND Pregnanediol Glucuronide, C27H44O8, also known as PDG (5β-Pregnan-3a,20a-diol 3-glucosiduronate) is the major metabolite of progesterone1-4. Progesterone is the hormone involved in the female menstrual cycle, gestation and embryogenesis of humans and other species. Progesterone belongs to a class of hormones called progestogens, and is the major naturally occurring human progestogen5,6. Progesterone is an essential regulator of human female reproductive function in the uterus, ovary, mammary gland and brain, and plays an important role in non-reproductive tissues such as the cardiovascular system, bone and the central nervous system. Progesterone action is conveyed by two isoforms of the nuclear progesterone receptor (PR), PRA and PRB. PRA and B are expressed in a variety of normal breast tissue from humans, rats and mice and is also expressed in breast cancer cells7,8. -

Steroid Metabolites Support Evidence of Autism As a Spectrum
behavioral sciences Article Steroid Metabolites Support Evidence of Autism as a Spectrum Benedikt Andreas Gasser 1,*, Johann Kurz 2, Bernhard Dick 1,3 and Markus Georg Mohaupt 1,4 1 Department of Clinical Research, University of Bern, 3010 Berne, Switzerland; [email protected] (B.D.); [email protected] (M.G.M.) 2 Intersci Research Association, Karl Morre Gasse 10, 8430 Leibnitz, Austria; [email protected] 3 Division of Nephrology/Hypertension, University of Bern, 3010 Berne, Switzerland 4 Teaching Hospital Internal Medicine, Lindenhofgruppe, 3006 Berne, Switzerland * Correspondence: [email protected] Received: 30 March 2019; Accepted: 6 May 2019; Published: 9 May 2019 Abstract: Objectives: It is common nowadays to refer to autism as a spectrum. Increased evidence of the involvement of steroid metabolites has been shown by the presence of stronger alterations in Kanner’s syndrome compared with Asperger syndrome. Methods: 24 h urine samples were collected from 20 boys with Asperger syndrome, 21 boys with Kanner’s syndrome, and identically sized control groups, each matched for age, weight, and height for comprehensive steroid hormone metabolite analysis via gas chromatography–mass spectrometry. Results: Higher levels of most steroid metabolites were detected in boys with Kanner’s syndrome and Asperger syndrome compared to their matched controls. These differences were more pronounced in affected individuals with Kanner’s syndrome versus Asperger syndrome. Furthermore, a specific and unique pattern of alteration of androsterone, etiocholanolone, progesterone, tetrahydrocortisone, and tetrahydrocortisol was identified in boys with Kanner’s syndrome and Asperger syndrome. Interestingly, in both matched samples, only androsterone, etiocholanolone, progesterone, tetrahydrocortisone, tetrahydrocortisol, and 5a-tetrahydrocortisol groups were positively correlated.