Studies on Steroid Fever II. Pyrogenic and Anti-Pyrogenic Activity in Vitro of Some Endogenous Steroids of Man
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
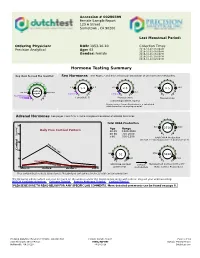
Hormone Testing Summary
Accession # 00280399 Female Sample Report 123 A Street Sometown , CA 90266 Last Menstrual Period: Ordering Physician: DOB: 1953-10-10 Collection Times: Precision Analytical Age: 63 2016-10-02 06:00AM 2016-10-02 08:00AM Gender: Female 2016-10-01 06:00PM 2016-10-01 10:00PM 2016-10-02 02:00AM Hormone Testing Summary Key (how to read the results): Sex Hormones See Pages 2 and 3 for a thorough breakdown of sex hormone metabolites opausa n l R e a m n e g 1.8 4.5 6.0 2.3 14.0 r 5.1 2.8 1.5 P e patient low limit high limit 20.0 result 0.2-0.7 0.3-2.0 Postmenopausal range Estradiol(E2) Progesterone Testosterone (Serum Equivalent, ng/mL) Progesterone Serum Equivalent is a calculated value based on urine pregnanediol. Adrenal Hormones See pages 4 and 5 for a more complete breakdown of adrenal hormones Total DHEA Production 300 500 3000 Age Range 2516 Daily Free Cortisol Pattern 20-39 1300-3000 240 40-60 750-2000 (ng/mg) >60 500-1200 Total DHEA Production (DHEAS + Etiocholanolone + Androsterone) 180 High Range Limit 120Cortisol 80 52 2750 5930 60 230 6500 Patient Values Low Range Limit 24hr Free Cortisol cortisol Metabolized Cortisol (THF+THE) 0 (A+B+C+D) metabolism (Total Cortisol Production) Waking (A) Morning (B) Afternoon (C) Night (D) Free cortisol best reflects tissue levels. Metabolized cortisol best reflects total cortisol production. The following videos (which can also be found on the website under the listed names along with others) may aid your understanding: DUTCH Complete Overview Estrogen Tutorial Female Androgen Tutorial Cortisol Tutorial PLEASE BE SURE TO READ BELOW FOR A NY SPECIFIC LA B COMMENTS. -

The Steroid Metabolome in Men with Mood and Anxiety Disorders
Physiol. Res. 64 (Suppl. 2): S275-S282, 2015 https://doi.org/10.33549/physiolres.933067 The Steroid Metabolome in Men With Mood and Anxiety Disorders M. DUŠKOVÁ1, M. HILL1, M. BIČÍKOVÁ1, M. ŠRÁMKOVÁ1, D. ŘÍPOVÁ2, P. MOHR2, L. STÁRKA1 1Institute of Endocrinology, Prague, Czech Republic, 2National Institute of Mental Health, Klecany, Czech Republic Received May 5, 2015 Accepted May 20, 2015 Summary many other hormones and various factors have been The mood and behavior of individuals result from an orchestra of identified as modulators of mood and behavior in such many factors. Among them steroids play an important role; disorders. Recent studies have described the role of brain- however, only several common hormones have been investigated derived neurotrophic factor (BDNF) (Pluchino et al. in this respect. It has been demonstrated that some steroid 2013, Numakawa et al. 2014), thyroid hormones (Duntas metabolites long considered merely the products of steroid and Mails 2013), inflammation (Halaris 2013), immunity hormone metabolism in fact possess considerable activity in the (Pitychoutis and Papadopoulou-Daifoti 2010), melatonin CNS. For this reason we studied the steroid metabolome (Boyce and Hopwood 2013), oxytocin and vasopressin including 50 analytes in 20 men with depression, 20 men with (Scantamburlo et al. 2009, Matsuzaki et al. 2012), the anxiety and 30 healthy controls. Significant differences were renin-angiotensin-aldosterone system (Franklin et al. found not only between controls and men with either depression 2012, Murck et al. 2012), the cannabinoid system or anxiety, but also between men with depression and anxiety. (Martykánová 2010, Gorzalka and Hill 2011, Smaga et Particularly striking were those steroids until now not generally al. -

Increased and Mistimed Sex Hormone Production in Night Shift Workers
Published OnlineFirst March 3, 2015; DOI: 10.1158/1055-9965.EPI-14-1271 Research Article Cancer Epidemiology, Biomarkers Increased and Mistimed Sex Hormone Production & Prevention in Night Shift Workers Kyriaki Papantoniou1,2,3,4, Oscar J. Pozo2, Ana Espinosa1,2,3,4, Josep Marcos2,3, Gemma Castano-Vinyals~ 1,2,3,4, Xavier Basagana~ 1,2,3,4, Elena Juanola Pages 5, Joan Mirabent6,7, Jordi Martín8, Patricia Such Faro9, Amparo Gasco Aparici10, Benita Middleton11, Debra J. Skene11, and Manolis Kogevinas1,2,3,4,12 Abstract Background: Night shift work has been associated with Results: Night workers had higher levels of total progestagens an increased risk for breast and prostate cancer. The effect [geometric mean ratio (GMR) 1.65; 95% confidence intervals of circadian disruption on sex steroid production is a pos- (CI), 1.17–2.32] and androgens (GMR: 1.44; 95% CI, 1.03–2.00), sible underlying mechanism, underinvestigated in hum- compared with day workers, after adjusting for potential con- ans. We have assessed daily rhythms of sex hormones founders. The increased sex hormone levels among night and melatonin in night and day shift workers of both shift workers were not related to the observed suppression of sexes. 6-sulfatoxymelatonin. Peak time of androgens was significantly Methods: We recruited 75 night and 42 day workers, ages later among night workers, compared with day workers (testos- 22 to 64 years, in different working settings. Participants terone: 12:14 hours; 10:06-14:48 vs. 08:35 hours; 06:52-10:46). collected urine samples from all voids over 24 hours on a Conclusions: We found increased levels of progestagens and working day. -

AOD Biennial Review
Introduction The Drug-Free Schools and Communities Act and Part 86 of the Education Department General Administrative Regulations (EDGAR) require each institution of higher education participating in Title IV student financial assistance to certify that it has developed and implemented a program to prevent the unlawful possession, use, and distribution of drugs and alcohol on campus and at institution-recognized events and activities. Annually, the institution must distribute to current students and employees written information about its drug and alcohol abuse prevention program, including information about related sanctions. The distribution plan must include provisions for sharing this information with new students and employees who join the institution at later points in the year as well. In addition, on a biennial basis, the institution must conduct a review to determine the effectiveness of its programs and prepare a report of the review’s findings. The report must be retained and made available to the US Department of Education upon request. Notre Dame of Maryland University (“NDMU” or “the University”) acknowledges these responsibilities, including the timely review of and reporting on the effectiveness of its prevention programs. NDMU is a private, Catholic university established in 1895 with the mission to educate leaders to transform the world. NDMU is a comprehensive university that is home to Maryland's only women's college and offers a wide variety of full and part-time undergraduate, graduate, doctoral, and certificate programs to women and men. The University enrolls approximately 2300 students and has Schools of Arts, Sciences, and Business; Education; Nursing; and Pharmacy. Additionally, the University offers programs for adult students fully online and at several locations throughout the State. -

Alteration of the Steroidogenesis in Boys with Autism Spectrum Disorders
Janšáková et al. Translational Psychiatry (2020) 10:340 https://doi.org/10.1038/s41398-020-01017-8 Translational Psychiatry ARTICLE Open Access Alteration of the steroidogenesis in boys with autism spectrum disorders Katarína Janšáková 1, Martin Hill 2,DianaČelárová1,HanaCelušáková1,GabrielaRepiská1,MarieBičíková2, Ludmila Máčová2 and Daniela Ostatníková1 Abstract The etiology of autism spectrum disorders (ASD) remains unknown, but associations between prenatal hormonal changes and ASD risk were found. The consequences of these changes on the steroidogenesis during a postnatal development are not yet well known. The aim of this study was to analyze the steroid metabolic pathway in prepubertal ASD and neurotypical boys. Plasma samples were collected from 62 prepubertal ASD boys and 24 age and sex-matched controls (CTRL). Eighty-two biomarkers of steroidogenesis were detected using gas-chromatography tandem-mass spectrometry. We observed changes across the whole alternative backdoor pathway of androgens synthesis toward lower level in ASD group. Our data indicate suppressed production of pregnenolone sulfate at augmented activities of CYP17A1 and SULT2A1 and reduced HSD3B2 activity in ASD group which is partly consistent with the results reported in older children, in whom the adrenal zona reticularis significantly influences the steroid levels. Furthermore, we detected the suppressed activity of CYP7B1 enzyme readily metabolizing the precursors of sex hormones on one hand but increased anti-glucocorticoid effect of 7α-hydroxy-DHEA via competition with cortisone for HSD11B1 on the other. The multivariate model found significant correlations between behavioral indices and circulating steroids. From dependent variables, the best correlation was found for the social interaction (28.5%). Observed changes give a space for their utilization as biomarkers while reveal the etiopathogenesis of ASD. -

Steroid Metabolites Support Evidence of Autism As a Spectrum
behavioral sciences Article Steroid Metabolites Support Evidence of Autism as a Spectrum Benedikt Andreas Gasser 1,*, Johann Kurz 2, Bernhard Dick 1,3 and Markus Georg Mohaupt 1,4 1 Department of Clinical Research, University of Bern, 3010 Berne, Switzerland; [email protected] (B.D.); [email protected] (M.G.M.) 2 Intersci Research Association, Karl Morre Gasse 10, 8430 Leibnitz, Austria; [email protected] 3 Division of Nephrology/Hypertension, University of Bern, 3010 Berne, Switzerland 4 Teaching Hospital Internal Medicine, Lindenhofgruppe, 3006 Berne, Switzerland * Correspondence: [email protected] Received: 30 March 2019; Accepted: 6 May 2019; Published: 9 May 2019 Abstract: Objectives: It is common nowadays to refer to autism as a spectrum. Increased evidence of the involvement of steroid metabolites has been shown by the presence of stronger alterations in Kanner’s syndrome compared with Asperger syndrome. Methods: 24 h urine samples were collected from 20 boys with Asperger syndrome, 21 boys with Kanner’s syndrome, and identically sized control groups, each matched for age, weight, and height for comprehensive steroid hormone metabolite analysis via gas chromatography–mass spectrometry. Results: Higher levels of most steroid metabolites were detected in boys with Kanner’s syndrome and Asperger syndrome compared to their matched controls. These differences were more pronounced in affected individuals with Kanner’s syndrome versus Asperger syndrome. Furthermore, a specific and unique pattern of alteration of androsterone, etiocholanolone, progesterone, tetrahydrocortisone, and tetrahydrocortisol was identified in boys with Kanner’s syndrome and Asperger syndrome. Interestingly, in both matched samples, only androsterone, etiocholanolone, progesterone, tetrahydrocortisone, tetrahydrocortisol, and 5a-tetrahydrocortisol groups were positively correlated. -

NON-CLINICAL REVIEW(S) DEPARTMENT of HEALTH & HUMAN SERVICES Food and Drug Administration
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 206089Orig1s000 NON-CLINICAL REVIEW(S) DEPARTMENT OF HEALTH & HUMAN SERVICES Food and Drug Administration PHARMACOLOGY/TOXICOLOGY MEMORANDUM CDER Stamp Date: September 27, 2018 NDA: 206089 Sponsor: Clarus Therapeutics, Inc. Drug: Testosterone undecanoate (JATENZO) Replacement therapy in adult males for conditions associated Indication: with a deficiency or absence of endogenous testosterone due to primary or hypogonadotropic hypogonadism Subject: Final Labeling Review Reviewer: Yangmee Shin, PhD Background: Clarus Therapeutics resubmitted NDA 206089 under a 505(b)(2) regulatory pathway following a 2nd Complete Response (CR) letter issued on March 22, 2018. NDA 206089 was first submitted on January 3, 2014 as a 505(b)(2) application. To support the nonclinical requirements of NDA via a 505(b)(2) pathway, the sponsor submitted published literature along with the findings of a 3-month oral toxicology study of Clarus’ oral testosterone undecanoate (TU) formulation in dogs. The sponsor also provided literature references to address ADME of TU by the oral route. The 3-month oral toxicology study in male dogs and relevant published literature were provided to support the use of borage oil as a novel excipient. The sponsor also supplied published literature regarding the fertility, pregnancy, and carcinogenicity of testosterone (T). Pharmacology and Toxicology recommended approval of the initial submission of NDA 206089 during the first cycle review. In the 1st resubmission to NDA 206089 on June 22, 2017, Clarus refiled the NDA as a 505(b)(1) application and provided nonclinical studies of oral TU including a 9-month oral toxicology study in male dogs, a battery of genotoxicity tests, a 6-month carcinogenicity study in Tg·rasH2 male mice, and a fertility study in male rats, upon agreement with the Division on November 19, 2015. -

DATA SHEET 1. REANDRON® 1000 (1000 Mg/4 Ml Solution for Injection)
DATA SHEET 1. REANDRON® 1000 (1000 mg/4 mL solution for injection) Reandron 1000, 1000 mg/ 4 mL solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ampoule/vial contains 1000 mg testosterone undecanoate (equivalent to 631.5 mg testosterone) in a 4 mL solution for injection (250 mg testosterone undecanoate/mL). Each mL solution for injection contains 250 mg testosterone undecanoate corresponding to 157.9 mg testosterone. For a full list of excipients, see Section 6.1. 3. PHARMACEUTICAL FORM Reandron 1000 is a clear, colourless to yellowish-brown oily solution for injection. Testosterone undecanoate is a white or off-white crystalline substance. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Testosterone replacement in primary and secondary male hypogonadism. 4.2 Dose and method of administration 4.2.1 Dose Reandron 1000 (1 ampoule/vial equivalent to 1000 mg testosterone undecanoate) is injected every 10 to 14 weeks for testosterone replacement, where testosterone deficiency has been confirmed by clinical features and biochemical tests. Injections with this frequency are capable of maintaining sufficient testosterone levels and do not lead to accumulation. 4.2.1.1 Start of Treatment Serum testosterone levels should be measured before start of treatment and during initiation of treatment. Depending on serum testosterone levels and clinical symptoms, the first injection interval may be reduced to a minimum of 6 weeks as compared to the recommended range of 10 to 14 weeks for maintenance. With this loading dose, sufficient steady-state testosterone levels may be achieved more rapidly. 4.2.1.2 Individualisation of Treatment The injection interval should remain within the recommended range of 10 to 14 weeks. -

Testosterone and Dihydrotestosterone in Normal Subjects, During Pregnancy, and in Hyperthyroidism
Metabolic Clearance Rate and Blood Production Rate of Testosterone and Dihydrotestosterone in Normal Subjects, during Pregnancy, and in Hyperthyroidism J. M. SAEz, M. G. FOREST, A. M. MoRErm, and J. BRnTND From the Unite' de Recherches Endocriniennes et Metaboliques chez l'Enfant (Institut National de la Sante et de la Recherche Medicale), H6pital Debrousse, Lyon 5', France A B S T R A C T The metabolic clearance rate (MCR) and (an adult with hyperthyroidism and two children) these blood production rate (BP) of testosterone (T) and dihy- two MCRs were greatly reduced compared to the normal drotestosterone (DHT), the conversion of plasma testos- females, but the conversion of testosterone into dihy- terone to plasma dihydrotestosterone, and the renal clear- drotestosterone was in the limits of normal male range ance of androstenedione, testosterone, and dihydrotestos- In the normal subjects the renal clearance of andros- terone have been studied in man. In eight normal men, the tenedione was greater than that of testosterone and di- MCRT (516±108 [SD] liters/m/day) was significantly hydrotestosterone. Less than 20% of the dihydrotestos- greater than the MCRDIT (391±71 [SD] liters/m'/day). terone and less than 10% of the androstenedione in the In seven females, the MCRT (304±53 [SD] liters/m'/day) urine is derived from the plasma dihydrotestosterone and was also greater than the MCRDET (209±45 [SD] liters/ androstenedione. m2/day) and both values were less than their respective values in men (P <0.001). In men the conversion of testosterone into dihydrotestosterone at 2.8±0.3% (SD) INTRODUCTION was greater than that found in females, 1.56±0.5% (SD) During the past few years increased attention has been (P < 0.001). -

Hydroxy-20-One in Squirrel Monkeys
Effective Erythropoiesis Induced by 5$-Pregnane-3$-Hydroxy-20-One in Squirrel Monkeys EMMANUEL C. BESA, Dov GORSHEIN, WILLIAM A. HArr, and FRANK H. GmuDNi From the Hematology Research Laboratory, Presbyterian-University of Pennsylvania Medical Center, Philadelphia, Pennsylvania 19104 A B S T R A C T The erythropoietic effect of 513-preg- poietic agent void of virilizing and salt-retaining prop- nane-3#-hydroxy-20-one, a naturally occurring steroid erties led us to search for an active metabolite that is metabolite of progesterone, was evaluated in the squirrel primarily erythropoietic. monkey by ferrokinetic studies,. red cell survival, and The reduction of the A4 unisaturationi of the A ring in blood volume measurements. The intramuscular adminis- testosterone results in the fornmation of 5a and 50 deriva- tration of this steroid in pharmacologic doses shortened tives (1. 2). The 5a derivatives are androgenic but the the 59Fe plasma clearance and increased the plasma iron 5V3 steroids were devoid of this property (3) in the turnover, thereby indicating an increase in erythro- rodent assay system. A group of naturally occurring poiesis. A normal 'Fe red cell uptake was observed, and metabolites with 5p-H configuration, in which the the bone marrow maturation time was not altered. Red junction between the first two rings (A: B cis) of the cell survival was the same in the treated and control steroid nucleus is highly angulated (Fig. 1), has been groups. After five weekly injections of the steroid, the found to have a potent ability to induce heme synthesis monkeys increased their red cell mass by 57%l. -

Non-Classic Disorder of Adrenal Steroidogenesis and Clinical Dilemmas in 21-Hydroxylase Deficiency Combined with Backdoor Androg
International Journal of Molecular Sciences Review Non-Classic Disorder of Adrenal Steroidogenesis and Clinical Dilemmas in 21-Hydroxylase Deficiency Combined with Backdoor Androgen Pathway. Mini-Review and Case Report Marta Sumi ´nska 1,* , Klaudia Bogusz-Górna 1, Dominika Wegner 1 and Marta Fichna 2 1 Department of Pediatric Diabetes and Obesity, Poznan University of Medical Sciences, 60-527 Poznan, Poland; [email protected] (K.B.-G.); [email protected] (D.W.) 2 Department of Endocrinology, Metabolism and Internal Medicine, Poznan University of Medical Sciences, 60-653 Poznan, Poland; mfi[email protected] * Correspondence: [email protected] Received: 3 June 2020; Accepted: 28 June 2020; Published: 29 June 2020 Abstract: Congenital adrenal hyperplasia (CAH) is the most common cause of primary adrenal insufficiency in children and adolescents. It comprises several clinical entities associated with mutations in genes, encoding enzymes involved in cortisol biosynthesis. The mutations lead to considerable (non-classic form) to almost complete (classic form) inhibition of enzymatic activity, reflected by different phenotypes and relevant biochemical alterations. Up to 95% cases of CAH are due to mutations in CYP21A2 gene and subsequent 21α-hydroxylase deficiency, characterized by impaired cortisol synthesis and adrenal androgen excess. In the past two decades an alternative (“backdoor”) pathway of androgens’ synthesis in which 5α-androstanediol, a precursor of the 5α-dihydrotestosterone, is produced from 17α-hydroxyprogesterone, with intermediate products 3α,5α-17OHP and androsterone, in the sequence and with roundabout of testosterone as an intermediate, was reported in some studies. This pathway is not always considered in the clinical assessment of patients with hyperandrogenism. -

Disappearance and Conjugation of Free Etiocholanolone Peter Berkeley Gregory Yale University
Yale University EliScholar – A Digital Platform for Scholarly Publishing at Yale Yale Medicine Thesis Digital Library School of Medicine 1963 Turnover rates: disappearance and conjugation of free etiocholanolone Peter Berkeley Gregory Yale University Follow this and additional works at: http://elischolar.library.yale.edu/ymtdl Recommended Citation Gregory, Peter Berkeley, "Turnover rates: disappearance and conjugation of free etiocholanolone" (1963). Yale Medicine Thesis Digital Library. 2673. http://elischolar.library.yale.edu/ymtdl/2673 This Open Access Thesis is brought to you for free and open access by the School of Medicine at EliScholar – A Digital Platform for Scholarly Publishing at Yale. It has been accepted for inclusion in Yale Medicine Thesis Digital Library by an authorized administrator of EliScholar – A Digital Platform for Scholarly Publishing at Yale. For more information, please contact [email protected]. Turnover Rates: Disappearance and Conjugation of Free Etiocholanolone Peter B. Gregory Yale School of Medicine 1963 Dedicated to George L, Cohn, Philip K» Bondy arid Morris Dillard for their invaluable assistance Digitized by the Internet Archive in 2017 with funding from The National Endowment for the Humanities and the Arcadia Fund https://archive.org/details/turnoverratesdisOOgreg 1 It is recognized that certain endogenous steroids xv^ith a 5-3eta configuration produce a febrile response in man, (1,2,3) Several of these compounds may have well known physiologic functions 5 the temperature rise in the luteal phase of the menstrual period, with the concomitant increase of progesterone and its pyrogenic metabolites, pregnanolone and pregnanediol, Other 5-Beta steroids such as 11-keto-pregnanolone, pregnane- dione, 21-hydro xy-pregnanedione, 11-Beta-hydroxy-etiocholanolone, etiocholanedione, and etiocholanolone have no known responsi¬ bility for temperature regulation in normal individuals, (3) In 1957, Kappas and his collaborators (4,5) administered etiocholanolone intramuscularly to normal volunteers.