Testosterone and Dihydrotestosterone in Normal Subjects, During Pregnancy, and in Hyperthyroidism
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
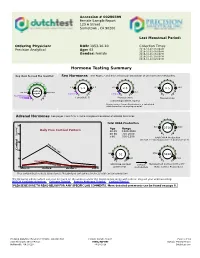
Hormone Testing Summary
Accession # 00280399 Female Sample Report 123 A Street Sometown , CA 90266 Last Menstrual Period: Ordering Physician: DOB: 1953-10-10 Collection Times: Precision Analytical Age: 63 2016-10-02 06:00AM 2016-10-02 08:00AM Gender: Female 2016-10-01 06:00PM 2016-10-01 10:00PM 2016-10-02 02:00AM Hormone Testing Summary Key (how to read the results): Sex Hormones See Pages 2 and 3 for a thorough breakdown of sex hormone metabolites opausa n l R e a m n e g 1.8 4.5 6.0 2.3 14.0 r 5.1 2.8 1.5 P e patient low limit high limit 20.0 result 0.2-0.7 0.3-2.0 Postmenopausal range Estradiol(E2) Progesterone Testosterone (Serum Equivalent, ng/mL) Progesterone Serum Equivalent is a calculated value based on urine pregnanediol. Adrenal Hormones See pages 4 and 5 for a more complete breakdown of adrenal hormones Total DHEA Production 300 500 3000 Age Range 2516 Daily Free Cortisol Pattern 20-39 1300-3000 240 40-60 750-2000 (ng/mg) >60 500-1200 Total DHEA Production (DHEAS + Etiocholanolone + Androsterone) 180 High Range Limit 120Cortisol 80 52 2750 5930 60 230 6500 Patient Values Low Range Limit 24hr Free Cortisol cortisol Metabolized Cortisol (THF+THE) 0 (A+B+C+D) metabolism (Total Cortisol Production) Waking (A) Morning (B) Afternoon (C) Night (D) Free cortisol best reflects tissue levels. Metabolized cortisol best reflects total cortisol production. The following videos (which can also be found on the website under the listed names along with others) may aid your understanding: DUTCH Complete Overview Estrogen Tutorial Female Androgen Tutorial Cortisol Tutorial PLEASE BE SURE TO READ BELOW FOR A NY SPECIFIC LA B COMMENTS. -

Aldrich FT-IR Collection Edition I Library
Aldrich FT-IR Collection Edition I Library Library Listing – 10,505 spectra This library is the original FT-IR spectral collection from Aldrich. It includes a wide variety of pure chemical compounds found in the Aldrich Handbook of Fine Chemicals. The Aldrich Collection of FT-IR Spectra Edition I library contains spectra of 10,505 pure compounds and is a subset of the Aldrich Collection of FT-IR Spectra Edition II library. All spectra were acquired by Sigma-Aldrich Co. and were processed by Thermo Fisher Scientific. Eight smaller Aldrich Material Specific Sub-Libraries are also available. Aldrich FT-IR Collection Edition I Index Compound Name Index Compound Name 3515 ((1R)-(ENDO,ANTI))-(+)-3- 928 (+)-LIMONENE OXIDE, 97%, BROMOCAMPHOR-8- SULFONIC MIXTURE OF CIS AND TRANS ACID, AMMONIUM SALT 209 (+)-LONGIFOLENE, 98+% 1708 ((1R)-ENDO)-(+)-3- 2283 (+)-MURAMIC ACID HYDRATE, BROMOCAMPHOR, 98% 98% 3516 ((1S)-(ENDO,ANTI))-(-)-3- 2966 (+)-N,N'- BROMOCAMPHOR-8- SULFONIC DIALLYLTARTARDIAMIDE, 99+% ACID, AMMONIUM SALT 2976 (+)-N-ACETYLMURAMIC ACID, 644 ((1S)-ENDO)-(-)-BORNEOL, 99% 97% 9587 (+)-11ALPHA-HYDROXY-17ALPHA- 965 (+)-NOE-LACTOL DIMER, 99+% METHYLTESTOSTERONE 5127 (+)-P-BROMOTETRAMISOLE 9590 (+)-11ALPHA- OXALATE, 99% HYDROXYPROGESTERONE, 95% 661 (+)-P-MENTH-1-EN-9-OL, 97%, 9588 (+)-17-METHYLTESTOSTERONE, MIXTURE OF ISOMERS 99% 730 (+)-PERSEITOL 8681 (+)-2'-DEOXYURIDINE, 99+% 7913 (+)-PILOCARPINE 7591 (+)-2,3-O-ISOPROPYLIDENE-2,3- HYDROCHLORIDE, 99% DIHYDROXY- 1,4- 5844 (+)-RUTIN HYDRATE, 95% BIS(DIPHENYLPHOSPHINO)BUT 9571 (+)-STIGMASTANOL -

(12) Patent Application Publication (10) Pub. No.: US 2006/0110428A1 De Juan Et Al
US 200601 10428A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0110428A1 de Juan et al. (43) Pub. Date: May 25, 2006 (54) METHODS AND DEVICES FOR THE Publication Classification TREATMENT OF OCULAR CONDITIONS (51) Int. Cl. (76) Inventors: Eugene de Juan, LaCanada, CA (US); A6F 2/00 (2006.01) Signe E. Varner, Los Angeles, CA (52) U.S. Cl. .............................................................. 424/427 (US); Laurie R. Lawin, New Brighton, MN (US) (57) ABSTRACT Correspondence Address: Featured is a method for instilling one or more bioactive SCOTT PRIBNOW agents into ocular tissue within an eye of a patient for the Kagan Binder, PLLC treatment of an ocular condition, the method comprising Suite 200 concurrently using at least two of the following bioactive 221 Main Street North agent delivery methods (A)-(C): Stillwater, MN 55082 (US) (A) implanting a Sustained release delivery device com (21) Appl. No.: 11/175,850 prising one or more bioactive agents in a posterior region of the eye so that it delivers the one or more (22) Filed: Jul. 5, 2005 bioactive agents into the vitreous humor of the eye; (B) instilling (e.g., injecting or implanting) one or more Related U.S. Application Data bioactive agents Subretinally; and (60) Provisional application No. 60/585,236, filed on Jul. (C) instilling (e.g., injecting or delivering by ocular ion 2, 2004. Provisional application No. 60/669,701, filed tophoresis) one or more bioactive agents into the Vit on Apr. 8, 2005. reous humor of the eye. Patent Application Publication May 25, 2006 Sheet 1 of 22 US 2006/0110428A1 R 2 2 C.6 Fig. -

The Steroid Metabolome in Men with Mood and Anxiety Disorders
Physiol. Res. 64 (Suppl. 2): S275-S282, 2015 https://doi.org/10.33549/physiolres.933067 The Steroid Metabolome in Men With Mood and Anxiety Disorders M. DUŠKOVÁ1, M. HILL1, M. BIČÍKOVÁ1, M. ŠRÁMKOVÁ1, D. ŘÍPOVÁ2, P. MOHR2, L. STÁRKA1 1Institute of Endocrinology, Prague, Czech Republic, 2National Institute of Mental Health, Klecany, Czech Republic Received May 5, 2015 Accepted May 20, 2015 Summary many other hormones and various factors have been The mood and behavior of individuals result from an orchestra of identified as modulators of mood and behavior in such many factors. Among them steroids play an important role; disorders. Recent studies have described the role of brain- however, only several common hormones have been investigated derived neurotrophic factor (BDNF) (Pluchino et al. in this respect. It has been demonstrated that some steroid 2013, Numakawa et al. 2014), thyroid hormones (Duntas metabolites long considered merely the products of steroid and Mails 2013), inflammation (Halaris 2013), immunity hormone metabolism in fact possess considerable activity in the (Pitychoutis and Papadopoulou-Daifoti 2010), melatonin CNS. For this reason we studied the steroid metabolome (Boyce and Hopwood 2013), oxytocin and vasopressin including 50 analytes in 20 men with depression, 20 men with (Scantamburlo et al. 2009, Matsuzaki et al. 2012), the anxiety and 30 healthy controls. Significant differences were renin-angiotensin-aldosterone system (Franklin et al. found not only between controls and men with either depression 2012, Murck et al. 2012), the cannabinoid system or anxiety, but also between men with depression and anxiety. (Martykánová 2010, Gorzalka and Hill 2011, Smaga et Particularly striking were those steroids until now not generally al. -

Anabolic-Androgenic Steroids in Horses: Natural Presence and Underlying Biomechanisms
ANABOLIC-ANDROGENIC STEROIDS IN HORSES: NATURAL PRESENCE AND UNDERLYING BIOMECHANISMS Anneleen Decloedt Dissertation submitted in the fulfilment of the requirements for the degree of Doctor of philosophy (PhD) in Veterinary Sciences, Faculty of Veterinary Medicine, Ghent University PROMOTER Prof. dr. ir. Lynn Vanhaecke Ghent University, Faculty of Veterinary Medicine Department of Veterinary Public Health and Food Safety Laboratory of Chemical Analysis MEMBERS OF THE READING COMMITTEE Prof. dr. James Scarth HFL Sport Science, Cambridgeshire, United-Kingdom Prof. dr. Peter Van Eenoo Ghent University, DoCoLab, Zwijnaarde, Belgium Prof. dr. Ann Van Soom Ghent University, Faculty of Veterinary Medicine, Merelbeke, Belgium MEMBERS OF THE EXAMINATION COMMITTEE Dr. Ludovic Bailly-Chouriberry Laboratoires des Courses Hippiques, Verrières-le-Buisson, France Dr. Leen Van Ginkel Wageningen University, RIKILT, Wageningen, The Netherlands Prof. dr. Myriam Hesta Ghent University, Faculty of Veterinary Medicine, Merelbeke, Belgium This work was funded by the Fédération Nationale des Courses Françaises (via the Laboratoire des Courses Hippiques) and executed at the Laboratory of Chemical Analysis (Faculty of Veterinary Medicine, Ghent University, Merelbeke). The author and the promoter give the authorisation to consult and to copy parts of this work for personal use only. Every other use is subject to the copyright laws. Permission to reproduce any material contained in this work should be obtained from the author. “The universe is full of magic, Just patiently waiting for our wits to grow sharper” TABLE OF CONTENTS TABLE OF CONTENTS Chapter I – General Introduction 1 1. Steroids 3 1.1 Chemical structure 1.2 (Steroid) hormones and their role in the endocrine system 1.3 Biosynthesis of steroid hormones 1.4 Anabolic-androgenic steroids (AAS) 1.5 Synthesis and absorption of the steroid precursor cholesterol 2. -

2013 House Judiciary Hb 1070
2013 HOUSE JUDICIARY HB 1070 2013 HOUSE STANDING COMMITTEE MINUTES House Judiciary Committee Prairie Room, State Capitol HB 1070 January 14, 2013 Job #17167 D Conference Committee � · Committee Clerk Signature A-. .;' /) .1� ' I A.J4-��'N'f?4<'71<P2/ I Explanation or reason for introduction of bill/resolution: Relating to the scheduling of controlled substances. Minutes: Chairman Koppelman: Opened the hearing on HB 1070. Mark Hardy, Assistant Executive Director of the NO State Board of Pharmacy: (See testimony #1and 2) He went over these handouts. Rep. Klemin: I don't see an emergency clause in the bill and there is none in the amendment. Did I miss it? Mark Hardy: I want to put an emergency clause on the bill. Rep. Klemin: I think you have to have another section at the end of the bill saying this is an emergency. Rep. Kretschmar: How often do these new drugs come out and should be on it? Mark Hardy: As far as the schedule 1 substances; it is a revolving door and we are always trying to stay in front of what the chemists and what the drug makers are doing. As far as schedule 2 through 5; it is a continuous thing through the DEA. When it becomes a federally controlled substance it takes precedence. Rep. Larson: You have not been aware of the bill I was sponsoring regarding synthetic drugs yet? Mark Hardy: No. The Attorney General briefed me on the Bill #1133. Rep. Larson: The reason for my bill is not get into all of the pharmaceutical names of the chemicals, but anybody that possess or manufacturers a analog in order to try and copy these drugs would be guilty of those offences without having to know the specific chemical compound that might be morphed by unscrupulous people trying to see these products. -

Increased and Mistimed Sex Hormone Production in Night Shift Workers
Published OnlineFirst March 3, 2015; DOI: 10.1158/1055-9965.EPI-14-1271 Research Article Cancer Epidemiology, Biomarkers Increased and Mistimed Sex Hormone Production & Prevention in Night Shift Workers Kyriaki Papantoniou1,2,3,4, Oscar J. Pozo2, Ana Espinosa1,2,3,4, Josep Marcos2,3, Gemma Castano-Vinyals~ 1,2,3,4, Xavier Basagana~ 1,2,3,4, Elena Juanola Pages 5, Joan Mirabent6,7, Jordi Martín8, Patricia Such Faro9, Amparo Gasco Aparici10, Benita Middleton11, Debra J. Skene11, and Manolis Kogevinas1,2,3,4,12 Abstract Background: Night shift work has been associated with Results: Night workers had higher levels of total progestagens an increased risk for breast and prostate cancer. The effect [geometric mean ratio (GMR) 1.65; 95% confidence intervals of circadian disruption on sex steroid production is a pos- (CI), 1.17–2.32] and androgens (GMR: 1.44; 95% CI, 1.03–2.00), sible underlying mechanism, underinvestigated in hum- compared with day workers, after adjusting for potential con- ans. We have assessed daily rhythms of sex hormones founders. The increased sex hormone levels among night and melatonin in night and day shift workers of both shift workers were not related to the observed suppression of sexes. 6-sulfatoxymelatonin. Peak time of androgens was significantly Methods: We recruited 75 night and 42 day workers, ages later among night workers, compared with day workers (testos- 22 to 64 years, in different working settings. Participants terone: 12:14 hours; 10:06-14:48 vs. 08:35 hours; 06:52-10:46). collected urine samples from all voids over 24 hours on a Conclusions: We found increased levels of progestagens and working day. -

Quick Determination of 105 Doping in Animal Derived Food By
QTRAP®质谱系统对动物源食品中105种兴奋剂的快速分析 检测 Quick Determination of 105 Doping in Animal – derived Food by QTRAP® System 杨总1,陈丹2,程海燕1,李立军1,郭立海1 Yang Zong1,Chen Dan2,Cheng Haiyan1,Li Lijun1,Guo Lihai1 1 SCIEX亚太应用支持中心,上海; 2 湖北省武汉食品化妆品检验所 1 SCIEX Asia Pacific Application Support Center, Shanghai, China; 2 Wuhan Institute for Food and Cosmetic Control, Hubei Province, China; Keywords:doping;SCIEX QTRAP® 4500 系统; LC-MS/ MS;quantitative analysis 引言 在现代畜牧养殖业中,一些不法分子长期使用各种饲料添加 剂和人工合成激素类化合物,造成动物源食品中的药物残留,最 终成为食源性兴奋剂的来源[1]。运动员食用含有某些药物残留的食 物,可导致其兴奋剂检测呈阳性。国家反兴奋剂中心和世界反兴 奋剂机构(WADA)等组织机构明确规定了兴奋剂的违禁药物的种 SCIEX ExionLC™液相和SCIEX QTRAP® 4500质谱系统 类,主要包含了β-受体激动剂、固醇类激素、糖皮质激素、利尿 剂、玉米赤霉醇类等等。总体而言,兴奋剂的作用主要是对身体或 者中枢神经系统起到选择性的作用,能促进肌肉组织快速增长、增 4. 建立好了105种兴奋剂EPI二级谱库,软件自动进行匹配二级质 强机体活力和敏捷度,还可使运动员疲劳减轻,自信心增强,注意 谱图,保证筛查结果准确可靠; 力集中[2]。因此在体育赛事过程中,为了保证赛事结果的公平、公 正,在运动员食品安全保障过程中,必须对食品中兴奋剂进行严格 5. 方法现成,省去开发方法的时间,提高了工作效率; 的监控。本文针对动物源食品中的常见105种兴奋剂,建立了采用 QTRAP®系统快速筛查和定量方法,该方法完全满足在体育赛事过 1. 实验方法 程中对兴奋剂监管的分析要求。 1.1 液相色谱条件 该方案的特点和优势 色谱柱:Phenomenex Kinetex 2.6µm F5 100 Å 150×3 µm 流动相:水相(水中含有5 mM乙酸铵和0.01%甲酸),有机 1. 方法覆盖面广,包含了常见五大类β-受体激动剂、固醇类激 相为乙腈,梯度洗脱 素、糖皮质激素、利尿剂、玉米赤霉醇类兴奋剂,共105种; 1.2 质谱条件 2. 本实验详细优化了前处理过程,考察了化合物的稳定性; 扫描模式:MRM-IDA-EPI,正负离子同时扫描,MRM离子对见 3. 实验采用MRM-IDA-EPI扫描实现一针进样同时定量和定性分 表1。 析,快速灵敏,满足国内外标准品的要求; RUO-MKT-02-9756-ZH-A p 1 离子源:ESI源;离子源参数:喷雾电压(IS):5500V(+) 2. 结果与讨论 /-4500V(-);离子源温度:600℃;气帘气(CUR):35psi;碰 2.1 前处理过程的优化 撞气(CAD):Medium;雾化气(GS1):55psi;辅助雾化气 (GS2):60psi 本实验详细优化了前处理过程,实验结果表明在动物源食品 中酸化乙腈的提取效率最高,绝大部分化合物回收率在70%以上 1.3 样品前处理过程 (见图1、图2、图3)。由于基质的复杂性,实验比较了5种不同 称取5g样品于50 mL离心管 → 加入10 mL乙酸铵缓冲溶液混 的SPE柱的净化效率,由图4可以看出,PRIME HLB 净化效果最优 异,回收率较高。 匀 → 加入β-葡萄糖醛甙酶进行酶解 → 加入酸化乙腈进行提取, 震荡混匀后加入NaCl和Mg2SO4 → 萃取之后高速离心取上清液采用 PRIME HLB进行净化 → 吹干后复溶上机测试。 100 80 60 40 20 0 酸化叔丁基甲醚 酸化乙腈 碱化乙腈 图1. -

Studies on Steroid Fever II. Pyrogenic and Anti-Pyrogenic Activity in Vitro of Some Endogenous Steroids of Man
Studies on steroid fever II. Pyrogenic and anti-pyrogenic activity in vitro of some endogenous steroids of man G. M. Dillard, Phyllis Bodel J Clin Invest. 1970;49(12):2418-2426. https://doi.org/10.1172/JCI106461. Research Article The pyrogenic properties of some C-19 and C-21 steroids were examined by in vitro incubation of human blood leukocytes with serum-buffer solutions of the steroids and injection of the 18-hr supernatants into rabbits. In previous studies this method demonstrated release of leukocyte endogenous pyrogen by etiocholanolone. With two exceptions, steroids known to cause fever in man, such as 11β-OH etiocholanolone and 3α-hydroxy-5β-pregnane-20-one were also pyrogenic in vitro. All steroids tested which are nonpyrogenic in man, such as androsterone, 3β-OH etiocholanolone, and 3α, 17α-dihydroxy-5β-pregnan-20-one were also nonpyrogenic in vitro. Solubility in aqueous solution did not correlate with pyrogenic capacity. Inhibition of pyrogen release from human leukocytes in vitro by hydrocortisone and estradiol was demonstrated. Hydrocortisone-treated leukocytes released less pyrogen than did normal leukocytes when stimulated either by etiocholanolone or by phagocytosis of heat-killed staphylococci. On the other hand, estradiol-treated blood leukocytes and mononuclear cells showed significant suppression of pyrogen release when phagocytosis, but not etiocholanolone, was used as the stimulus. When blood cells were incubated with progesterone, greater than normal amounts of pyrogen were released following phagocytosis, and the inhibiting effect of estradiol could be partially reversed. Neither estradiol nor hydrocortisone appeared to act on rabbit leukocytes. These studies indicate that a variety of naturally-occurring steroids may alter pyrogen release from leukocytes. -

(12) Patent Application Publication (10) Pub. No.: US 2008/0063698 A1 Hartwig (43) Pub
US 2008 OO63698A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0063698 A1 Hartwig (43) Pub. Date: Mar. 13, 2008 (54) CRYSTALLIZATION INHIBITION OF DRUGS now abandoned, which is a continuation of applica IN TRANSIDERMAL DRUG DELIVERY tion No. 10/010,342, filed on Dec. 5, 2001, now SYSTEMIS AND METHODS OF USE abandoned. (75) Inventor: Rod Lawson Hartwig, Plantation, FL (60) Provisional application No. 60/251.294, filed on Dec. (US) 5, 2000. Correspondence Address: Publication Classification Noven Pharmaceuticals, Inc. Jay G. Kolman, Esq. (51) Int. Cl. 11960 S.W. 144 Street A6II 3/56 (2006.01) A6F 3/00 (2006.01) Miami, FL 33186 (US) A6IP 43/00 (2006.01) (73) Assignee: NOVEN PHARMACEUTICALS, A6F 3/02 (2006.01) INC. (52) U.S. Cl. ........................... 424/448; 424/449; 514/170 (57) ABSTRACT Appl. No.: 11/897,189 (21) The invention relates to compositions and methods for (22) Filed: Aug. 29, 2007 making a transdermal drug delivery system capable of achieving substantially zero-order kinetics for delivery of Related U.S. Application Data the active agent over a period of time in excess of 24 hours and at least 72 hours, comprising a pharmaceutically accept (63) Continuation of application No. 10/751,152, filed on able active agent carrier and a rosin ester which provides a Jan. 2, 2004, now abandoned, which is a continuation crystal inhibiting and drug stabilizing effect on the active of application No. 10/353,624, filed on Jan. 29, 2003, agents incorporated therein. 13 2 2 Patent Application Publication Mar. -

Title 21–Food and Drugs
Title 21–Food and Drugs (This book contains part 1300 to End) Part CHAPTER II—Drug Enforcement Administration, Depart- ment of Justice .................................................................. 1301 CHAPTER III—Office of National Drug Control Policy ............ 1401 1 VerDate Mar<15>2010 11:22 May 06, 2014 Jkt 232078 PO 00000 Frm 00011 Fmt 8008 Sfmt 8008 Y:\SGML\232078.XXX 232078 ehiers on DSK2VPTVN1PROD with CFR VerDate Mar<15>2010 11:22 May 06, 2014 Jkt 232078 PO 00000 Frm 00012 Fmt 8008 Sfmt 8008 Y:\SGML\232078.XXX 232078 ehiers on DSK2VPTVN1PROD with CFR CHAPTER II—DRUG ENFORCEMENT ADMINISTRATION, DEPARTMENT OF JUSTICE Part Page 1300 Definitions .............................................................. 5 1301 Registration of manufacturers, distributors, and dispensers of controlled substances ..................... 21 1302 Labeling and packaging requirements for con- trolled substances ................................................ 54 1303 Quotas ..................................................................... 56 1304 Records and reports of registrants .......................... 64 1305 Orders for schedule I and II controlled substances 82 1306 Prescriptions ........................................................... 90 1307 Miscellaneous .......................................................... 102 1308 Schedules of controlled substances ......................... 105 1309 Registration of manufacturers, distributors, im- porters and exporters of list I chemicals .............. 129 1310 Records and reports of listed -

AOD Biennial Review
Introduction The Drug-Free Schools and Communities Act and Part 86 of the Education Department General Administrative Regulations (EDGAR) require each institution of higher education participating in Title IV student financial assistance to certify that it has developed and implemented a program to prevent the unlawful possession, use, and distribution of drugs and alcohol on campus and at institution-recognized events and activities. Annually, the institution must distribute to current students and employees written information about its drug and alcohol abuse prevention program, including information about related sanctions. The distribution plan must include provisions for sharing this information with new students and employees who join the institution at later points in the year as well. In addition, on a biennial basis, the institution must conduct a review to determine the effectiveness of its programs and prepare a report of the review’s findings. The report must be retained and made available to the US Department of Education upon request. Notre Dame of Maryland University (“NDMU” or “the University”) acknowledges these responsibilities, including the timely review of and reporting on the effectiveness of its prevention programs. NDMU is a private, Catholic university established in 1895 with the mission to educate leaders to transform the world. NDMU is a comprehensive university that is home to Maryland's only women's college and offers a wide variety of full and part-time undergraduate, graduate, doctoral, and certificate programs to women and men. The University enrolls approximately 2300 students and has Schools of Arts, Sciences, and Business; Education; Nursing; and Pharmacy. Additionally, the University offers programs for adult students fully online and at several locations throughout the State.