DATA SHEET 1. REANDRON® 1000 (1000 Mg/4 Ml Solution for Injection)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
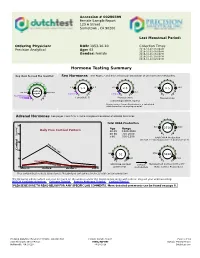
Hormone Testing Summary
Accession # 00280399 Female Sample Report 123 A Street Sometown , CA 90266 Last Menstrual Period: Ordering Physician: DOB: 1953-10-10 Collection Times: Precision Analytical Age: 63 2016-10-02 06:00AM 2016-10-02 08:00AM Gender: Female 2016-10-01 06:00PM 2016-10-01 10:00PM 2016-10-02 02:00AM Hormone Testing Summary Key (how to read the results): Sex Hormones See Pages 2 and 3 for a thorough breakdown of sex hormone metabolites opausa n l R e a m n e g 1.8 4.5 6.0 2.3 14.0 r 5.1 2.8 1.5 P e patient low limit high limit 20.0 result 0.2-0.7 0.3-2.0 Postmenopausal range Estradiol(E2) Progesterone Testosterone (Serum Equivalent, ng/mL) Progesterone Serum Equivalent is a calculated value based on urine pregnanediol. Adrenal Hormones See pages 4 and 5 for a more complete breakdown of adrenal hormones Total DHEA Production 300 500 3000 Age Range 2516 Daily Free Cortisol Pattern 20-39 1300-3000 240 40-60 750-2000 (ng/mg) >60 500-1200 Total DHEA Production (DHEAS + Etiocholanolone + Androsterone) 180 High Range Limit 120Cortisol 80 52 2750 5930 60 230 6500 Patient Values Low Range Limit 24hr Free Cortisol cortisol Metabolized Cortisol (THF+THE) 0 (A+B+C+D) metabolism (Total Cortisol Production) Waking (A) Morning (B) Afternoon (C) Night (D) Free cortisol best reflects tissue levels. Metabolized cortisol best reflects total cortisol production. The following videos (which can also be found on the website under the listed names along with others) may aid your understanding: DUTCH Complete Overview Estrogen Tutorial Female Androgen Tutorial Cortisol Tutorial PLEASE BE SURE TO READ BELOW FOR A NY SPECIFIC LA B COMMENTS. -

The Steroid Metabolome in Men with Mood and Anxiety Disorders
Physiol. Res. 64 (Suppl. 2): S275-S282, 2015 https://doi.org/10.33549/physiolres.933067 The Steroid Metabolome in Men With Mood and Anxiety Disorders M. DUŠKOVÁ1, M. HILL1, M. BIČÍKOVÁ1, M. ŠRÁMKOVÁ1, D. ŘÍPOVÁ2, P. MOHR2, L. STÁRKA1 1Institute of Endocrinology, Prague, Czech Republic, 2National Institute of Mental Health, Klecany, Czech Republic Received May 5, 2015 Accepted May 20, 2015 Summary many other hormones and various factors have been The mood and behavior of individuals result from an orchestra of identified as modulators of mood and behavior in such many factors. Among them steroids play an important role; disorders. Recent studies have described the role of brain- however, only several common hormones have been investigated derived neurotrophic factor (BDNF) (Pluchino et al. in this respect. It has been demonstrated that some steroid 2013, Numakawa et al. 2014), thyroid hormones (Duntas metabolites long considered merely the products of steroid and Mails 2013), inflammation (Halaris 2013), immunity hormone metabolism in fact possess considerable activity in the (Pitychoutis and Papadopoulou-Daifoti 2010), melatonin CNS. For this reason we studied the steroid metabolome (Boyce and Hopwood 2013), oxytocin and vasopressin including 50 analytes in 20 men with depression, 20 men with (Scantamburlo et al. 2009, Matsuzaki et al. 2012), the anxiety and 30 healthy controls. Significant differences were renin-angiotensin-aldosterone system (Franklin et al. found not only between controls and men with either depression 2012, Murck et al. 2012), the cannabinoid system or anxiety, but also between men with depression and anxiety. (Martykánová 2010, Gorzalka and Hill 2011, Smaga et Particularly striking were those steroids until now not generally al. -

Increased and Mistimed Sex Hormone Production in Night Shift Workers
Published OnlineFirst March 3, 2015; DOI: 10.1158/1055-9965.EPI-14-1271 Research Article Cancer Epidemiology, Biomarkers Increased and Mistimed Sex Hormone Production & Prevention in Night Shift Workers Kyriaki Papantoniou1,2,3,4, Oscar J. Pozo2, Ana Espinosa1,2,3,4, Josep Marcos2,3, Gemma Castano-Vinyals~ 1,2,3,4, Xavier Basagana~ 1,2,3,4, Elena Juanola Pages 5, Joan Mirabent6,7, Jordi Martín8, Patricia Such Faro9, Amparo Gasco Aparici10, Benita Middleton11, Debra J. Skene11, and Manolis Kogevinas1,2,3,4,12 Abstract Background: Night shift work has been associated with Results: Night workers had higher levels of total progestagens an increased risk for breast and prostate cancer. The effect [geometric mean ratio (GMR) 1.65; 95% confidence intervals of circadian disruption on sex steroid production is a pos- (CI), 1.17–2.32] and androgens (GMR: 1.44; 95% CI, 1.03–2.00), sible underlying mechanism, underinvestigated in hum- compared with day workers, after adjusting for potential con- ans. We have assessed daily rhythms of sex hormones founders. The increased sex hormone levels among night and melatonin in night and day shift workers of both shift workers were not related to the observed suppression of sexes. 6-sulfatoxymelatonin. Peak time of androgens was significantly Methods: We recruited 75 night and 42 day workers, ages later among night workers, compared with day workers (testos- 22 to 64 years, in different working settings. Participants terone: 12:14 hours; 10:06-14:48 vs. 08:35 hours; 06:52-10:46). collected urine samples from all voids over 24 hours on a Conclusions: We found increased levels of progestagens and working day. -

Pharmaceuticals and Endocrine Active Chemicals in Minnesota Lakes
Pharmaceuticals and Endocrine Active Chemicals in Minnesota Lakes May 2013 Authors Mark Ferrey Contributors/acknowledgements The MPCA is reducing printing and mailing costs This report contains the results of a study that by using the Internet to distribute reports and characterizes the presence of unregulated information to wider audience. Visit our website contaminants in Minnesota’s lakes. The study for more information. was made possible through funding by the MPCA reports are printed on 100 percent post- Minnesota Clean Water Fund and by funding by consumer recycled content paper manufactured the U.S. Environmental Protection Agency without chlorine or chlorine derivatives. (EPA), which facilitated the sampling of lakes for this study. The Minnesota Pollution Control Agency (MPCA) thanks the following for assistance and advice in designing and carrying out this study: Steve Heiskary, Pam Anderson, Dereck Richter, Lee Engel, Amy Garcia, Will Long, Jesse Anderson, Ben Larson, and Kelly O’Hara for the long hours of sampling for this study. Cynthia Tomey, Kirsten Anderson, and Richard Grace of Axys Analytical Labs for the expert help in developing the list of analytes for this study and logistics to make it a success. Minnesota Pollution Control Agency 520 Lafayette Road North | Saint Paul, MN 55155-4194 | www.pca.state.mn.us | 651-296-6300 Toll free 800-657-3864 | TTY 651-282-5332 This report is available in alternative formats upon request, and online at www.pca.state.mn.us. Document number: tdr-g1-16 Contents Contents ........................................................................................................................................... -

Gender-Affirming Hormone Therapy
GENDER-AFFIRMING HORMONE THERAPY Julie Thompson, PA-C Medical Director of Trans Health, Fenway Health March 2020 fenwayhealth.org GOALS AND OBJECTIVES 1. Review process of initiating hormone therapy through the informed consent model 2. Provide an overview of masculinizing and feminizing hormone therapy 3. Review realistic expectations and benefits of hormone therapy vs their associated risks 4. Discuss recommendations for monitoring fenwayhealth.org PROTOCOLS AND STANDARDS OF CARE fenwayhealth.org WPATH STANDARDS OF CARE, 2011 The criteria for hormone therapy are as follows: 1. Well-documented, persistent (at least 6mo) gender dysphoria 2. Capacity to make a fully informed decision and to consent for treatment 3. Age of majority in a given country 4. If significant medical or mental health concerns are present, they must be reasonably well controlled fenwayhealth.org INFORMED CONSENT MODEL ▪ Requires healthcare provider to ▪ Effectively communicate benefits, risks and alternatives of treatment to patient ▪ Assess that the patient is able to understand and consent to the treatment ▪ Informed consent model does not preclude mental health care! ▪ Recognizes that prescribing decision ultimately rests with clinical judgment of provider working together with the patient ▪ Recognizes patient autonomy and empowers self-agency ▪ Decreases barriers to medically necessary care fenwayhealth.org INITIAL VISITS ▪ Review history of gender experience and patient’s goals ▪ Document prior hormone use ▪ Assess appropriateness for gender affirming medical -

Studies on Steroid Fever II. Pyrogenic and Anti-Pyrogenic Activity in Vitro of Some Endogenous Steroids of Man
Studies on steroid fever II. Pyrogenic and anti-pyrogenic activity in vitro of some endogenous steroids of man G. M. Dillard, Phyllis Bodel J Clin Invest. 1970;49(12):2418-2426. https://doi.org/10.1172/JCI106461. Research Article The pyrogenic properties of some C-19 and C-21 steroids were examined by in vitro incubation of human blood leukocytes with serum-buffer solutions of the steroids and injection of the 18-hr supernatants into rabbits. In previous studies this method demonstrated release of leukocyte endogenous pyrogen by etiocholanolone. With two exceptions, steroids known to cause fever in man, such as 11β-OH etiocholanolone and 3α-hydroxy-5β-pregnane-20-one were also pyrogenic in vitro. All steroids tested which are nonpyrogenic in man, such as androsterone, 3β-OH etiocholanolone, and 3α, 17α-dihydroxy-5β-pregnan-20-one were also nonpyrogenic in vitro. Solubility in aqueous solution did not correlate with pyrogenic capacity. Inhibition of pyrogen release from human leukocytes in vitro by hydrocortisone and estradiol was demonstrated. Hydrocortisone-treated leukocytes released less pyrogen than did normal leukocytes when stimulated either by etiocholanolone or by phagocytosis of heat-killed staphylococci. On the other hand, estradiol-treated blood leukocytes and mononuclear cells showed significant suppression of pyrogen release when phagocytosis, but not etiocholanolone, was used as the stimulus. When blood cells were incubated with progesterone, greater than normal amounts of pyrogen were released following phagocytosis, and the inhibiting effect of estradiol could be partially reversed. Neither estradiol nor hydrocortisone appeared to act on rabbit leukocytes. These studies indicate that a variety of naturally-occurring steroids may alter pyrogen release from leukocytes. -

Gender-Affirming Hormone Therapy
GENDER-AFFIRMING HORMONE THERAPY Julie Thompson, PA-C Medical Director of Trans Health Fenway Health November 2019 fenwayhealth.org GOALS AND OBJECTIVES 1.Review process of initiating hormone therapy through the informed consent model 2.Provide an overview of masculinizing and feminizing hormone therapy 3.Review realistic expectations and benefits of hormone therapy vs their associated risks 4.Discuss recommendations for monitoring fenwayhealth.org PROTOCOLS AND STANDARDS OF CARE fenwayhealth.org WPATH STANDARDS OF CARE, 2011 The criteria for hormone therapy are as follows: 1. Well-documented, persistent (at least 6mo) gender dysphoria 2. Capacity to make a fully informed decision and to consent for treatment 3. Age of majority in a given country 4. If significant medical or mental health concerns are present, they must be reasonably well controlled fenwayhealth.org INFORMED CONSENT MODEL ▪ Requires healthcare provider to ▪ Effectively communicate benefits, risks and alternatives of treatment to patient ▪ Assess that the patient is able to understand and consent to the treatment ▪ Informed consent model does not preclude mental health care! ▪ Recognizes that prescribing decision ultimately rests with clinical judgment of provider working together with the patient ▪ Recognizes patient autonomy and empowers self-agency ▪ Decreases barriers to medically necessary care fenwayhealth.org INITIAL VISITS ▪ Review history of gender experience ▪ Document prior hormone use ▪ Review patient goals ▪ Assess appropriateness for gender affirming medical -

Pharmaceutical and Veterinary Compounds and Metabolites
PHARMACEUTICAL AND VETERINARY COMPOUNDS AND METABOLITES High quality reference materials for analytical testing of pharmaceutical and veterinary compounds and metabolites. lgcstandards.com/drehrenstorfer [email protected] LGC Quality | ISO 17034 | ISO/IEC 17025 | ISO 9001 PHARMACEUTICAL AND VETERINARY COMPOUNDS AND METABOLITES What you need to know Pharmaceutical and veterinary medicines are essential for To facilitate the fair trade of food, and to ensure a consistent human and animal welfare, but their use can leave residues and evidence-based approach to consumer protection across in both the food chain and the environment. In a 2019 survey the globe, the Codex Alimentarius Commission (“Codex”) was of EU member states, the European Food Safety Authority established in 1963. Codex is a joint agency of the FAO (Food (EFSA) found that the number one food safety concern was and Agriculture Office of the United Nations) and the WHO the misuse of antibiotics, hormones and steroids in farm (World Health Organisation). It is responsible for producing animals. This is, in part, related to the issue of growing antibiotic and maintaining the Codex Alimentarius: a compendium of resistance in humans as a result of their potential overuse in standards, guidelines and codes of practice relating to food animals. This level of concern and increasing awareness of safety. The legal framework for the authorisation, distribution the risks associated with veterinary residues entering the food and control of Veterinary Medicinal Products (VMPs) varies chain has led to many regulatory bodies increasing surveillance from country to country, but certain common principles activities for pharmaceutical and veterinary residues in food and apply which are described in the Codex guidelines. -

FDA Briefing Document NDA 206089 Testosterone Undecanoate
FDA Briefing Document NDA 206089 Testosterone Undecanoate (proposed trade name Jatenzo) For replacement therapy in adult males for conditions associated with a deficiency or absence of endogenous testosterone. Bone, Reproductive, and Urologic Drugs Advisory Committee (BRUDAC) Meeting January 9, 2018 Division of Bone, Reproductive, and Urologic Products Office of New Drugs Division of Clinical Pharmacology 3 Office of Clinical Pharmacology Center for Drug Evaluation and Research 1 DISCLAIMER STATEMENT The attached package contains background information prepared by the Food and Drug Administration (FDA) for the panel members of the advisory committee. The FDA background package often contains assessments and/or conclusions and recommendations written by individual FDA reviewers. Such conclusions and recommendations do not necessarily represent the final position of the individual reviewers, nor do they necessarily represent the final position of the Review Division or Office. We have brought a new drug application (NDA 206089) for testosterone undecanoate oral capsules (proposed trade name, JATENZO), intended for replacement therapy in adult males for conditions associated with a deficiency or absence of endogenous testosterone sponsored by Clarus Therapeutics, Inc, to this Advisory Committee in order to gain the Committee’s insights and opinions. The background package may not include all issues relevant to the final regulatory recommendation and instead is intended to focus on issues identified by the Agency for discussion by the advisory committee. The FDA will not issue a final determination on the issues at hand until input from the advisory committee process has been considered and all reviews have been finalized. The final determination may be affected by issues not discussed at the advisory committee meeting. -

AOD Biennial Review
Introduction The Drug-Free Schools and Communities Act and Part 86 of the Education Department General Administrative Regulations (EDGAR) require each institution of higher education participating in Title IV student financial assistance to certify that it has developed and implemented a program to prevent the unlawful possession, use, and distribution of drugs and alcohol on campus and at institution-recognized events and activities. Annually, the institution must distribute to current students and employees written information about its drug and alcohol abuse prevention program, including information about related sanctions. The distribution plan must include provisions for sharing this information with new students and employees who join the institution at later points in the year as well. In addition, on a biennial basis, the institution must conduct a review to determine the effectiveness of its programs and prepare a report of the review’s findings. The report must be retained and made available to the US Department of Education upon request. Notre Dame of Maryland University (“NDMU” or “the University”) acknowledges these responsibilities, including the timely review of and reporting on the effectiveness of its prevention programs. NDMU is a private, Catholic university established in 1895 with the mission to educate leaders to transform the world. NDMU is a comprehensive university that is home to Maryland's only women's college and offers a wide variety of full and part-time undergraduate, graduate, doctoral, and certificate programs to women and men. The University enrolls approximately 2300 students and has Schools of Arts, Sciences, and Business; Education; Nursing; and Pharmacy. Additionally, the University offers programs for adult students fully online and at several locations throughout the State. -

(12) United States Patent (10) Patent No.: US 6,284,263 B1 Place (45) Date of Patent: Sep
USOO6284263B1 (12) United States Patent (10) Patent No.: US 6,284,263 B1 Place (45) Date of Patent: Sep. 4, 2001 (54) BUCCAL DRUG ADMINISTRATION IN THE 4,755,386 7/1988 Hsiao et al. TREATMENT OF FEMALE SEXUAL 4,764,378 8/1988 Keith et al.. DYSFUNCTION 4,877,774 10/1989 Pitha et al.. 5,135,752 8/1992 Snipes. 5,190,967 3/1993 Riley. (76) Inventor: Virgil A. Place, P.O. Box 44555-10 5,346,701 9/1994 Heiber et al. Ala Kahua, Kawaihae, HI (US) 96743 5,516,523 5/1996 Heiber et al. 5,543,154 8/1996 Rork et al. ........................ 424/133.1 (*) Notice: Subject to any disclaimer, the term of this 5,639,743 6/1997 Kaswan et al. patent is extended or adjusted under 35 6,180,682 1/2001 Place. U.S.C. 154(b) by 0 days. * cited by examiner (21) Appl. No.: 09/626,772 Primary Examiner Thurman K. Page ASSistant Examiner-Rachel M. Bennett (22) Filed: Jul. 27, 2000 (74) Attorney, Agent, or Firm-Dianne E. Reed; Reed & Related U.S. Application Data ASSciates (62) Division of application No. 09/237,713, filed on Jan. 26, (57) ABSTRACT 1999, now Pat. No. 6,117,446. A buccal dosage unit is provided for administering a com (51) Int. Cl. ............................. A61F 13/02; A61 K9/20; bination of Steroidal active agents to a female individual. A61K 47/30 The novel buccal drug delivery Systems may be used in (52) U.S. Cl. .......................... 424/435; 424/434; 424/464; female hormone replacement therapy, in female 514/772.3 contraception, to treat female Sexual dysfunction, and to treat or prevent a variety of conditions and disorders which (58) Field of Search .................................... -

Steroids and Other Appearance and Performance Enhancing Drugs (Apeds) Research Report
Research Report Revised Febrero 2018 Steroids and Other Appearance and Performance Enhancing Drugs (APEDs) Research Report Table of Contents Steroids and Other Appearance and Performance Enhancing Drugs (APEDs) Research Report Introduction What are the different types of APEDs? What is the history of anabolic steroid use? Who uses anabolic steroids? Why are anabolic steroids misused? How are anabolic steroids used? What are the side effects of anabolic steroid misuse? How does anabolic steroid misuse affect behavior? What are the risks of anabolic steroid use in teens? How do anabolic steroids work in the brain? Are anabolic steroids addictive? How are anabolic steroids tested in athletes? What can be done to prevent steroid misuse? What treatments are effective for anabolic steroid misuse? Where can I get further information about steroids? References Page 1 Steroids and Other Appearance and Performance Enhancing Drugs (APEDs) Research Report Esta publicación está disponible para su uso y puede ser reproducida, en su totalidad, sin pedir autorización al NIDA. Se agradece la citación de la fuente, de la siguiente manera: Fuente: Instituto Nacional sobre el Abuso de Drogas; Institutos Nacionales de la Salud; Departamento de Salud y Servicios Humanos de los Estados Unidos. Introduction Appearance and performance enhancing drugs (APEDs) are most often used by males to improve appearance by building muscle mass or to enhance athletic performance. Although they may directly and indirectly have effects on a user’s mood, they do not produce a euphoric high, which makes APEDs distinct from other drugs such as cocaine, heroin, and marijuana. However, users may develop a substance use disorder, defined as continued use despite adverse consequences.