Anabolic Steroid Abuse
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
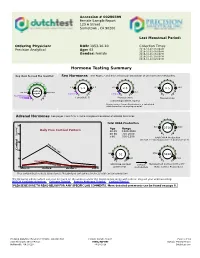
Hormone Testing Summary
Accession # 00280399 Female Sample Report 123 A Street Sometown , CA 90266 Last Menstrual Period: Ordering Physician: DOB: 1953-10-10 Collection Times: Precision Analytical Age: 63 2016-10-02 06:00AM 2016-10-02 08:00AM Gender: Female 2016-10-01 06:00PM 2016-10-01 10:00PM 2016-10-02 02:00AM Hormone Testing Summary Key (how to read the results): Sex Hormones See Pages 2 and 3 for a thorough breakdown of sex hormone metabolites opausa n l R e a m n e g 1.8 4.5 6.0 2.3 14.0 r 5.1 2.8 1.5 P e patient low limit high limit 20.0 result 0.2-0.7 0.3-2.0 Postmenopausal range Estradiol(E2) Progesterone Testosterone (Serum Equivalent, ng/mL) Progesterone Serum Equivalent is a calculated value based on urine pregnanediol. Adrenal Hormones See pages 4 and 5 for a more complete breakdown of adrenal hormones Total DHEA Production 300 500 3000 Age Range 2516 Daily Free Cortisol Pattern 20-39 1300-3000 240 40-60 750-2000 (ng/mg) >60 500-1200 Total DHEA Production (DHEAS + Etiocholanolone + Androsterone) 180 High Range Limit 120Cortisol 80 52 2750 5930 60 230 6500 Patient Values Low Range Limit 24hr Free Cortisol cortisol Metabolized Cortisol (THF+THE) 0 (A+B+C+D) metabolism (Total Cortisol Production) Waking (A) Morning (B) Afternoon (C) Night (D) Free cortisol best reflects tissue levels. Metabolized cortisol best reflects total cortisol production. The following videos (which can also be found on the website under the listed names along with others) may aid your understanding: DUTCH Complete Overview Estrogen Tutorial Female Androgen Tutorial Cortisol Tutorial PLEASE BE SURE TO READ BELOW FOR A NY SPECIFIC LA B COMMENTS. -

Differential Effects of Hormones on Cellular Metabolism in Keratoconus
www.nature.com/scientificreports OPEN Differential Effects of Hormones on Cellular Metabolism in Keratoconus In Vitro Received: 27 October 2016 Tina B McKay1, Jesper Hjortdal2, Henrik Sejersen2 & Dimitrios Karamichos1,3 Accepted: 18 January 2017 Keratoconus (KC) is a corneal thinning disease with an onset commonly immediately post-puberty Published: 17 February 2017 and stabilization by 40 to 50 years of age. The role of hormones in regulating corneal tissue structure in homeostatic and pathological conditions is unknown. Our group recently linked altered hormone levels to KC. Our current study sought to investigate and delineate the effects of exogenous hormones, such as androgen, luteotropin, and estrogen, on corneal stroma bioenergetics. We utilized our established 3D in vitro model to characterize the effects of DHEA, prolactin, 17β-estradiol on insulin- growth factor-1 and -2 (IGF-1, -2) signaling and metabolic function in primary corneal fibroblasts from healthy controls (HCFs) and KC patients (HKCs). Our data showed that exogenous DHEA significantly downregulated IGF-1 and its receptor in both HCFs and HKCs with HKCs showing consistently lower basal pentose phosphate flux. Prolactin caused no significant change in IGF-1 levels and an increase in IGF-2 in HKCs correlating with an increase in ATP and NADH levels. 17β-estradiol led to a significant upregulation in pentose phosphate flux and glycolytic intermediates in HCFs. Our results identified hormone-specific responses regulated in HKCs compared to HCFs revealing a novel role for hormones on bioenergetics in KC. Sex hormones play a functional role in regulating growth and reproduction, systemic metabolism, and cellular differentiation and functionality1–3. -

Intramuscular Injections of Male Pheromone 5Α-Androstenol Change the Secretory Ovarian Function in Gilts During Sexual Maturation
Vol. 3, No. 3 241 ORIGINAL RESEARCH Intramuscular injections of male pheromone 5α-androstenol change the secretory ovarian function in gilts during sexual maturation Stanisława Stefańczyk-Krzymowska1, Tadeusz Krzymowski, Barbara Wąsowska, Barbara Jana, Jarosław Słomiński Division of Reproductive Endocrinology and Pathophysiology, Institute of Animal Reproduction and Food Research of the Polish Academy of Sciences, Olsztyn, Poland Received: 8 September 2003; accepted: 4 November 2003 SUMMARY In addition to the standard olfactory pathway typical for signaling phero- mones, the existence of a humoral pathway for the priming action of phero- mones has been earlier postulated. In this study in vivo experiment was performed to establish whether intramuscular injections of boar pheromone, 5α-androstenol (5α-androst-16-en-3-ol), might change the development and secretory function of the ovarian follicles during sexual maturation of gilts. Gilts from groups I (n=15) and II (n=13) received androstenol (10 μg/gilt/injection; i.m.) three times a week from day 192 to 234 of age. Similar, control gilts (group C; n=13) received saline. Additionally, the na- sal cavity of animals from group II was irrigated with zinc sulfate solution to depress olfactory function. The reproductive organs and follicular fl uid 1Corresponding author: Institute of Animal Reproduction and Food Research of the Polish Academy of Sciences, Tuwima 10, 10-747 Olsztyn, Poland; E-mail: [email protected] Copyright © 2003 by the Society for Biology of Reproduction 242 Male pheromonepheromone in gilts were collected on day 240 of age. There were no signifi cant differences among groups concerning the weight of the ovary and uterus, the length of the uterine horns and intensity of cytochrome P450scc and P450arom im- munoexpression. -

Hormonal Treatment Strategies Tailored to Non-Binary Transgender Individuals
Journal of Clinical Medicine Review Hormonal Treatment Strategies Tailored to Non-Binary Transgender Individuals Carlotta Cocchetti 1, Jiska Ristori 1, Alessia Romani 1, Mario Maggi 2 and Alessandra Daphne Fisher 1,* 1 Andrology, Women’s Endocrinology and Gender Incongruence Unit, Florence University Hospital, 50139 Florence, Italy; [email protected] (C.C); jiska.ristori@unifi.it (J.R.); [email protected] (A.R.) 2 Department of Experimental, Clinical and Biomedical Sciences, Careggi University Hospital, 50139 Florence, Italy; [email protected]fi.it * Correspondence: fi[email protected] Received: 16 April 2020; Accepted: 18 May 2020; Published: 26 May 2020 Abstract: Introduction: To date no standardized hormonal treatment protocols for non-binary transgender individuals have been described in the literature and there is a lack of data regarding their efficacy and safety. Objectives: To suggest possible treatment strategies for non-binary transgender individuals with non-standardized requests and to emphasize the importance of a personalized clinical approach. Methods: A narrative review of pertinent literature on gender-affirming hormonal treatment in transgender persons was performed using PubMed. Results: New hormonal treatment regimens outside those reported in current guidelines should be considered for non-binary transgender individuals, in order to improve psychological well-being and quality of life. In the present review we suggested the use of hormonal and non-hormonal compounds, which—based on their mechanism of action—could be used in these cases depending on clients’ requests. Conclusion: Requests for an individualized hormonal treatment in non-binary transgender individuals represent a future challenge for professionals managing transgender health care. For each case, clinicians should balance the benefits and risks of a personalized non-standardized treatment, actively involving the person in decisions regarding hormonal treatment. -

The Steroid Metabolome in Men with Mood and Anxiety Disorders
Physiol. Res. 64 (Suppl. 2): S275-S282, 2015 https://doi.org/10.33549/physiolres.933067 The Steroid Metabolome in Men With Mood and Anxiety Disorders M. DUŠKOVÁ1, M. HILL1, M. BIČÍKOVÁ1, M. ŠRÁMKOVÁ1, D. ŘÍPOVÁ2, P. MOHR2, L. STÁRKA1 1Institute of Endocrinology, Prague, Czech Republic, 2National Institute of Mental Health, Klecany, Czech Republic Received May 5, 2015 Accepted May 20, 2015 Summary many other hormones and various factors have been The mood and behavior of individuals result from an orchestra of identified as modulators of mood and behavior in such many factors. Among them steroids play an important role; disorders. Recent studies have described the role of brain- however, only several common hormones have been investigated derived neurotrophic factor (BDNF) (Pluchino et al. in this respect. It has been demonstrated that some steroid 2013, Numakawa et al. 2014), thyroid hormones (Duntas metabolites long considered merely the products of steroid and Mails 2013), inflammation (Halaris 2013), immunity hormone metabolism in fact possess considerable activity in the (Pitychoutis and Papadopoulou-Daifoti 2010), melatonin CNS. For this reason we studied the steroid metabolome (Boyce and Hopwood 2013), oxytocin and vasopressin including 50 analytes in 20 men with depression, 20 men with (Scantamburlo et al. 2009, Matsuzaki et al. 2012), the anxiety and 30 healthy controls. Significant differences were renin-angiotensin-aldosterone system (Franklin et al. found not only between controls and men with either depression 2012, Murck et al. 2012), the cannabinoid system or anxiety, but also between men with depression and anxiety. (Martykánová 2010, Gorzalka and Hill 2011, Smaga et Particularly striking were those steroids until now not generally al. -

Drug Testing Program
DRUG TESTING PROGRAM Copyright © 2021 CrossFit, LLC. All Rights Reserved. CrossFit is a registered trademark ® of CrossFit, LLC. 2021 DRUG TESTING PROGRAM 2021 DRUG TESTING CONTENTS 1. DRUG-FREE COMPETITION 2. ATHLETE CONSENT 3. DRUG TESTING 4. IN-COMPETITION/OUT-OF-COMPETITION DRUG TESTING 5. REGISTERED ATHLETE TESTING POOL (OUT-OF-COMPETITION DRUG TESTING) 6. REMOVAL FROM TESTING POOL/RETIREMENT 6A. REMOVAL FROM TESTING POOL/WATCH LIST 7. TESTING POOL REQUIREMENTS FOLLOWING A SANCTION 8. DRUG TEST NOTIFICATION AND ADMINISTRATION 9. SPECIMEN ANALYSIS 10. REPORTING RESULTS 11. DRUG TESTING POLICY VIOLATIONS 12. ENFORCEMENT/SANCTIONS 13. APPEALS PROCESS 14. LEADERBOARD DISPLAY 15. EDUCATION 16. DIETARY SUPPLEMENTS 17. TRANSGENDER POLICY 18. THERAPEUTIC USE EXEMPTION APPENDIX A: 2020-2021 CROSSFIT BANNED SUBSTANCE CLASSES APPENDIX B: CROSSFIT URINE TESTING PROCEDURES - (IN-COMPETITION) APPENDIX C: TUE APPLICATION REQUIREMENTS Drug Testing Policy V4 Copyright © 2021 CrossFit, LLC. All Rights Reserved. CrossFit is a registered trademark ® of CrossFit, LLC. [ 2 ] 2021 DRUG TESTING PROGRAM 2021 DRUG TESTING 1. DRUG-FREE COMPETITION As the world’s definitive test of fitness, CrossFit Games competitions stand not only as testaments to the athletes who compete but to the training methodologies they use. In this arena, a true and honest comparison of training practices and athletic capacity is impossible without a level playing field. Therefore, the use of banned performance-enhancing substances is prohibited. Even the legal use of banned substances, such as physician-prescribed hormone replacement therapy or some over-the-counter performance-enhancing supplements, has the potential to compromise the integrity of the competition and must be disallowed. With the health, safety, and welfare of the athletes, and the integrity of our sport as top priorities, CrossFit, LLC has adopted the following Drug Testing Policy to ensure the validity of the results achieved in competition. -

Effect of the Acute Stress Response on Foraging Behavior in Mountain White-Crowned Sparrows, Zonotrichia Leucophrys Sarah C
Claremont Colleges Scholarship @ Claremont Scripps Senior Theses Scripps Student Scholarship 2015 Effect of the Acute Stress Response on Foraging Behavior in Mountain White-Crowned Sparrows, Zonotrichia Leucophrys Sarah C. Osborne Scripps College Recommended Citation Osborne, Sarah C., "Effect of the Acute Stress Response on Foraging Behavior in Mountain White-Crowned Sparrows, Zonotrichia Leucophrys" (2015). Scripps Senior Theses. Paper 573. http://scholarship.claremont.edu/scripps_theses/573 This Open Access Senior Thesis is brought to you for free and open access by the Scripps Student Scholarship at Scholarship @ Claremont. It has been accepted for inclusion in Scripps Senior Theses by an authorized administrator of Scholarship @ Claremont. For more information, please contact [email protected]. Effect of the Acute Stress Response on Foraging Behavior in Mountain White-crowned Sparrows, Zonotrichia leucophrys A Thesis Presented By Sarah Osborne To the Keck Science Department Of Claremont McKenna, Pitzer, and Scripps College In partial fulfillment of The degree of Bachelor of Arts Senior Thesis in Biology December 8th, 2014 Osborne 1 Table of Contents Abstract………………………………………………………………………pp. 2 Chapter 1: A Review of Hormonal Effects in Vertebrates…………………..pp. 2-10 Hormones and Behavior.…………………………………………….pp. 3-4 Hormones’ Relationship to Allostasis……………………………….pp. 4-5 Glucocorticoids and Behavior……………………………………….pp. 6-7 Glucocorticoids’ Relationship to Stress.…………………………….pp. 8-9 Free CORT, Bound CORT, and CBGs………………………………pp. 10 Chapter 2: Research Study: Effect of Acute Stress on Foraging Behavior….pp. 11-20 Introduction………………………………………………………….pp. 11-12 Methods……………………………………………………………...pp. 12-14 Results……………………………………………………………….pp. 14-18 Discussion…………………………………………………………....pp. 19-21 Chapter 3: Implications and Future Directions………………………………pp. 21-24 Acknowledgements…………………………………………………………..pp. 24 Appendix……………………………………………………………………..pp. -

Increased and Mistimed Sex Hormone Production in Night Shift Workers
Published OnlineFirst March 3, 2015; DOI: 10.1158/1055-9965.EPI-14-1271 Research Article Cancer Epidemiology, Biomarkers Increased and Mistimed Sex Hormone Production & Prevention in Night Shift Workers Kyriaki Papantoniou1,2,3,4, Oscar J. Pozo2, Ana Espinosa1,2,3,4, Josep Marcos2,3, Gemma Castano-Vinyals~ 1,2,3,4, Xavier Basagana~ 1,2,3,4, Elena Juanola Pages 5, Joan Mirabent6,7, Jordi Martín8, Patricia Such Faro9, Amparo Gasco Aparici10, Benita Middleton11, Debra J. Skene11, and Manolis Kogevinas1,2,3,4,12 Abstract Background: Night shift work has been associated with Results: Night workers had higher levels of total progestagens an increased risk for breast and prostate cancer. The effect [geometric mean ratio (GMR) 1.65; 95% confidence intervals of circadian disruption on sex steroid production is a pos- (CI), 1.17–2.32] and androgens (GMR: 1.44; 95% CI, 1.03–2.00), sible underlying mechanism, underinvestigated in hum- compared with day workers, after adjusting for potential con- ans. We have assessed daily rhythms of sex hormones founders. The increased sex hormone levels among night and melatonin in night and day shift workers of both shift workers were not related to the observed suppression of sexes. 6-sulfatoxymelatonin. Peak time of androgens was significantly Methods: We recruited 75 night and 42 day workers, ages later among night workers, compared with day workers (testos- 22 to 64 years, in different working settings. Participants terone: 12:14 hours; 10:06-14:48 vs. 08:35 hours; 06:52-10:46). collected urine samples from all voids over 24 hours on a Conclusions: We found increased levels of progestagens and working day. -

Part I Biopharmaceuticals
1 Part I Biopharmaceuticals Translational Medicine: Molecular Pharmacology and Drug Discovery First Edition. Edited by Robert A. Meyers. © 2018 Wiley-VCH Verlag GmbH & Co. KGaA. Published 2018 by Wiley-VCH Verlag GmbH & Co. KGaA. 3 1 Analogs and Antagonists of Male Sex Hormones Robert W. Brueggemeier The Ohio State University, Division of Medicinal Chemistry and Pharmacognosy, College of Pharmacy, Columbus, Ohio 43210, USA 1Introduction6 2 Historical 6 3 Endogenous Male Sex Hormones 7 3.1 Occurrence and Physiological Roles 7 3.2 Biosynthesis 8 3.3 Absorption and Distribution 12 3.4 Metabolism 13 3.4.1 Reductive Metabolism 14 3.4.2 Oxidative Metabolism 17 3.5 Mechanism of Action 19 4 Synthetic Androgens 24 4.1 Current Drugs on the Market 24 4.2 Therapeutic Uses and Bioassays 25 4.3 Structure–Activity Relationships for Steroidal Androgens 26 4.3.1 Early Modifications 26 4.3.2 Methylated Derivatives 26 4.3.3 Ester Derivatives 27 4.3.4 Halo Derivatives 27 4.3.5 Other Androgen Derivatives 28 4.3.6 Summary of Structure–Activity Relationships of Steroidal Androgens 28 4.4 Nonsteroidal Androgens, Selective Androgen Receptor Modulators (SARMs) 30 4.5 Absorption, Distribution, and Metabolism 31 4.6 Toxicities 32 Translational Medicine: Molecular Pharmacology and Drug Discovery First Edition. Edited by Robert A. Meyers. © 2018 Wiley-VCH Verlag GmbH & Co. KGaA. Published 2018 by Wiley-VCH Verlag GmbH & Co. KGaA. 4 Analogs and Antagonists of Male Sex Hormones 5 Anabolic Agents 32 5.1 Current Drugs on the Market 32 5.2 Therapeutic Uses and Bioassays -

Anabolic Steroids
epartment .D of .S Ju U s t ic U.S. Department of Justice e . n o Drug Enforcement Administration i t a r t is Office of Diversion Control D in Laws and penalties for anabolic r m ug d E t A steroid abuse (cont’d) nforcemen an individual’s first drug offense. The maximum www.dea.gov penalty for trafficking is five years in prison and a For more information: fine of $250,000 if this is the individual’s first felony drug offense. If this is the second felony drug offense, the maximum period of imprisonment and Please contact your nearest the maximum fine both double. The period of DEA office imprisonment and the amount of fine are enhanced Or if the offense involves the distribution of an anabolic Visit one of our Internet Websites: steroid and a masking agent or if the distribution is to an athlete. In addition, enhanced penalties exist www.DEAdiversion.usdoj.gov for any athletic coach who uses his/her position to Or influence an athlete to use an anabolic steroid. While www.dea.gov the above listed penalties are for federal offenses, individual states have also implemented fines and penalties for illegal use of anabolic steroids. The International Olympic Committee (IOC), National Collegiate Athletic Association (NCAA), and many professional sports leagues (e.g. Major League Baseball, National Basketball Association, National Football League , and National Hockey League) have banned the use of steroids by athletes, both because of their potential dangerous side effects and they give the user an unfair advantage. -

Studies on Steroid Fever II. Pyrogenic and Anti-Pyrogenic Activity in Vitro of Some Endogenous Steroids of Man
Studies on steroid fever II. Pyrogenic and anti-pyrogenic activity in vitro of some endogenous steroids of man G. M. Dillard, Phyllis Bodel J Clin Invest. 1970;49(12):2418-2426. https://doi.org/10.1172/JCI106461. Research Article The pyrogenic properties of some C-19 and C-21 steroids were examined by in vitro incubation of human blood leukocytes with serum-buffer solutions of the steroids and injection of the 18-hr supernatants into rabbits. In previous studies this method demonstrated release of leukocyte endogenous pyrogen by etiocholanolone. With two exceptions, steroids known to cause fever in man, such as 11β-OH etiocholanolone and 3α-hydroxy-5β-pregnane-20-one were also pyrogenic in vitro. All steroids tested which are nonpyrogenic in man, such as androsterone, 3β-OH etiocholanolone, and 3α, 17α-dihydroxy-5β-pregnan-20-one were also nonpyrogenic in vitro. Solubility in aqueous solution did not correlate with pyrogenic capacity. Inhibition of pyrogen release from human leukocytes in vitro by hydrocortisone and estradiol was demonstrated. Hydrocortisone-treated leukocytes released less pyrogen than did normal leukocytes when stimulated either by etiocholanolone or by phagocytosis of heat-killed staphylococci. On the other hand, estradiol-treated blood leukocytes and mononuclear cells showed significant suppression of pyrogen release when phagocytosis, but not etiocholanolone, was used as the stimulus. When blood cells were incubated with progesterone, greater than normal amounts of pyrogen were released following phagocytosis, and the inhibiting effect of estradiol could be partially reversed. Neither estradiol nor hydrocortisone appeared to act on rabbit leukocytes. These studies indicate that a variety of naturally-occurring steroids may alter pyrogen release from leukocytes. -

Adverse Effects of Anabolic-Androgenic Steroids: a Literature Review
healthcare Review Adverse Effects of Anabolic-Androgenic Steroids: A Literature Review Giuseppe Davide Albano 1,†, Francesco Amico 1,†, Giuseppe Cocimano 1, Aldo Liberto 1, Francesca Maglietta 2, Massimiliano Esposito 1, Giuseppe Li Rosi 3, Nunzio Di Nunno 4, Monica Salerno 1,‡ and Angelo Montana 1,*,‡ 1 Legal Medicine, Department of Medical, Surgical and Advanced Technologies, “G.F. Ingrassia”, University of Catania, 95123 Catania, Italy; [email protected] (G.D.A.); [email protected] (F.A.); [email protected] (G.C.); [email protected] (A.L.); [email protected] (M.E.); [email protected] (M.S.) 2 Department of Clinical and Experimental Medicine, University of Foggia, 71122 Foggia, Italy; [email protected] 3 Department of Law, Criminology, Magna Graecia University of Catanzaro, 88100 Catanzaro, Italy; [email protected] 4 Department of History, Society and Studies on Humanity, University of Salento, 73100 Lecce, Italy; [email protected] * Correspondence: [email protected]; Tel.: +39-095-3782153 † These authors contributed equally to this work. ‡ These authors share last authorship. Abstract: Anabolic-androgenic steroids (AASs) are a large group of molecules including endoge- nously produced androgens, such as testosterone, as well as synthetically manufactured derivatives. AAS use is widespread due to their ability to improve muscle growth for aesthetic purposes and athletes’ performance, minimizing androgenic effects. AAS use is very popular and 1–3% of US inhabitants have been estimated to be AAS users. However, AASs have side effects, involving all organs, tissues and body functions, especially long-term toxicity involving the cardiovascular system Citation: Albano, G.D.; Amico, F.; and the reproductive system, thereby, their abuse is considered a public health issue.